Pfizer and BioNTech get U.S. FDA approval for Omicron KP.2-adapted COVID-19 vaccine
Pfizer Inc. (NYSE: PFE, "Pfizer") and BioNTech SE (Nasdaq: BNTX, "BioNTech") revealed that the U.S. Food and Drug Administration ("FDA") has authorized the supplemental Biologics License Application for those aged 12 and above (COMIRNATY® (COVID-19 Vaccine, mRNA)), and granted emergency use authorization for those aged 6 months to 11 years (Pfizer-BioNTech COVID-19 Vaccine) for their Omicron KP.2-adapted 2024-2025 Formula COVID-19 vaccine. This season’s vaccine is intended as a single dose for most individuals aged 5 and above. Individuals aged 5 and older who have specific types of immunocompromise and have been previously vaccinated with Pfizer and BioNTech COVID-19 vaccines, or children under 5 who have not yet completed a three-dose series with previous versions of a COVID-19 vaccine, may be eligible for additional doses.
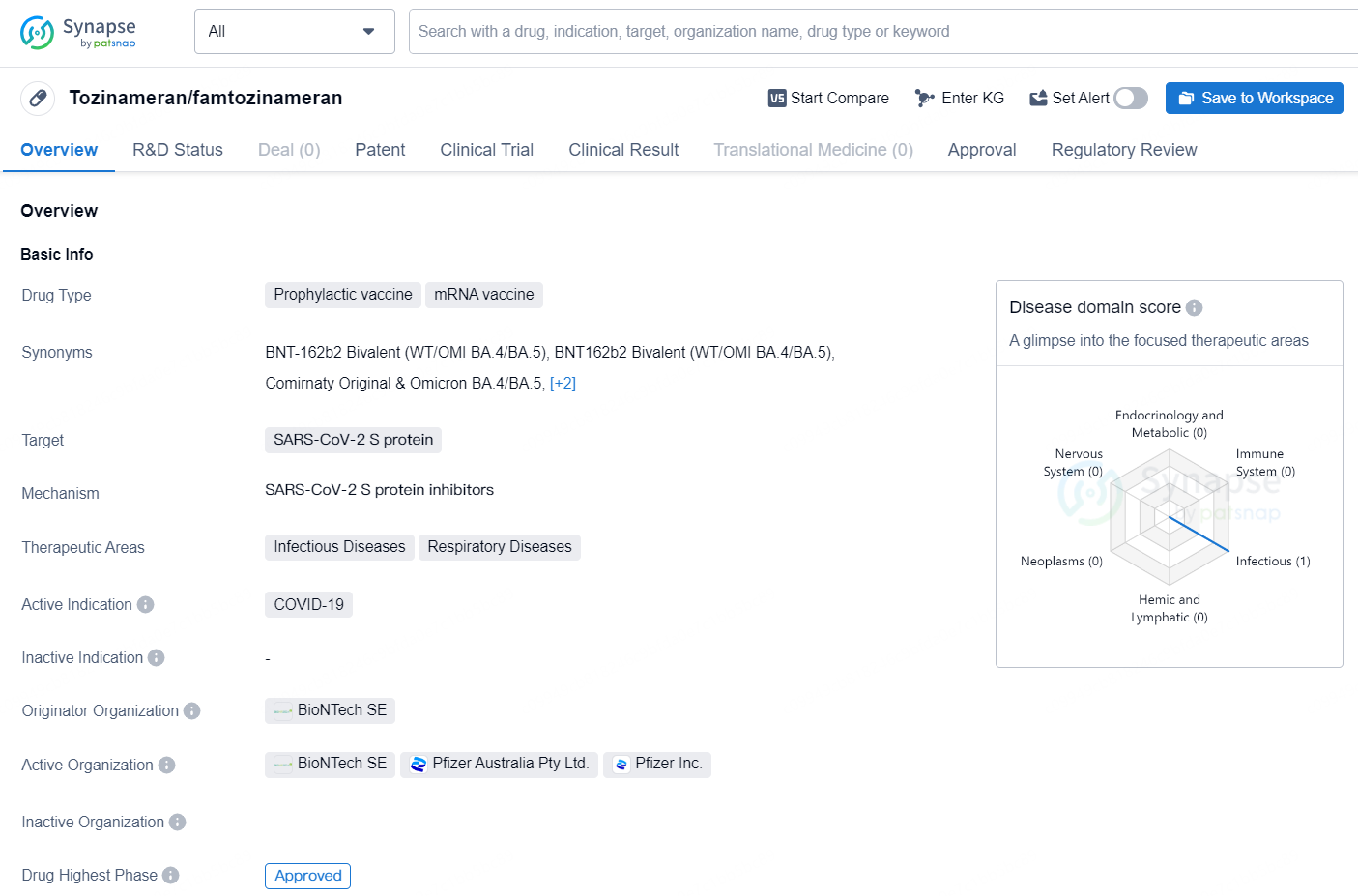
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This Phase 2 research is a randomized, double-blind, placebo-controlled, biopsy-guided trial unfolding at roughly 12 U.S. locations. Approximately 68 obese or overweight individuals (BMI ≥25kg/m2) with biopsy-confirmed MASH or MASLD will be included. Participants will be randomly assigned in a 1:1 ratio to receive either 40 mg of DD01 or a placebo weekly for 48 weeks. The primary goal of the study is to assess the proportion of participants who attain at least a 30% reduction in liver fat, as measured by MRI-PDFF, from baseline to Week 12. Secondary and exploratory goals will evaluate additional safety and efficacy factors, such as MASH resolution, fibrosis improvement, HbA1c levels, and body weight, with treatment continuing throughout the 48-week span.
Previously, D&D reported positive safety and efficacy results for DD01 in a Phase 1 multiple ascending dose (MAD) study involving overweight or obese patients with type 2 diabetes (T2D) and MASLD. DD01 was generally safe and well-tolerated at doses up to 80 mg weekly. Within just 4 weeks of treatment, up to 100% of patients experienced over a 30% reduction in liver fat measured by MRI-PDFF, with a mean relative reduction exceeding 50% in liver fat content, based on a pooled analysis of the 40 mg and 80 mg doses. These rapid improvements in steatosis were also correlated with reduced HbA1c levels in diabetic participants, as well as lowered liver AST/ALT and serum lipid levels. Throughout the 4-week treatment duration, subjects taking 40 and 80 mg of DD01 showed modest weight loss, which was not observed in those given a placebo.
These clinical findings are supported by preclinical studies in obese mice and primates, which showed that DD01 treatment was more effective in reducing liver fat and promoting weight loss compared to either dietary modifications or GLP-1 treatment alone. The lipolytic effects of glucagon are preserved and balanced with the anorectic and anti-diabetic properties of GLP-1, making this a true dual-pathway therapy. Preclinical models also showed MASH resolution, with notable reductions in liver steatosis, lobular inflammation, hepatocyte ballooning, and fibrosis. The US FDA has granted Fast Track designation to DD01 for the treatment of adults with MASLD/MASH.
“We are excited to start the DD01 Phase 2 study, which marks a significant milestone in the development of DD01 for MASH treatment,” said Seulki Lee, Ph.D., President and CEO of D&D Pharmatech. “Recent clinical data suggest that dual activation of GLP-1 and liver-directed glucagon receptors could be more effective in treating MASH compared to GLP-1 analogs without glucagon agonism. Considering that DD01 has already demonstrated rapid and substantial reductions in hepatic steatosis along with positive effects on glucose regulation within just 4 weeks of treatment, we aim to achieve meaningful rates of MASH resolution and fibrosis improvement in our Phase 2 trial.”
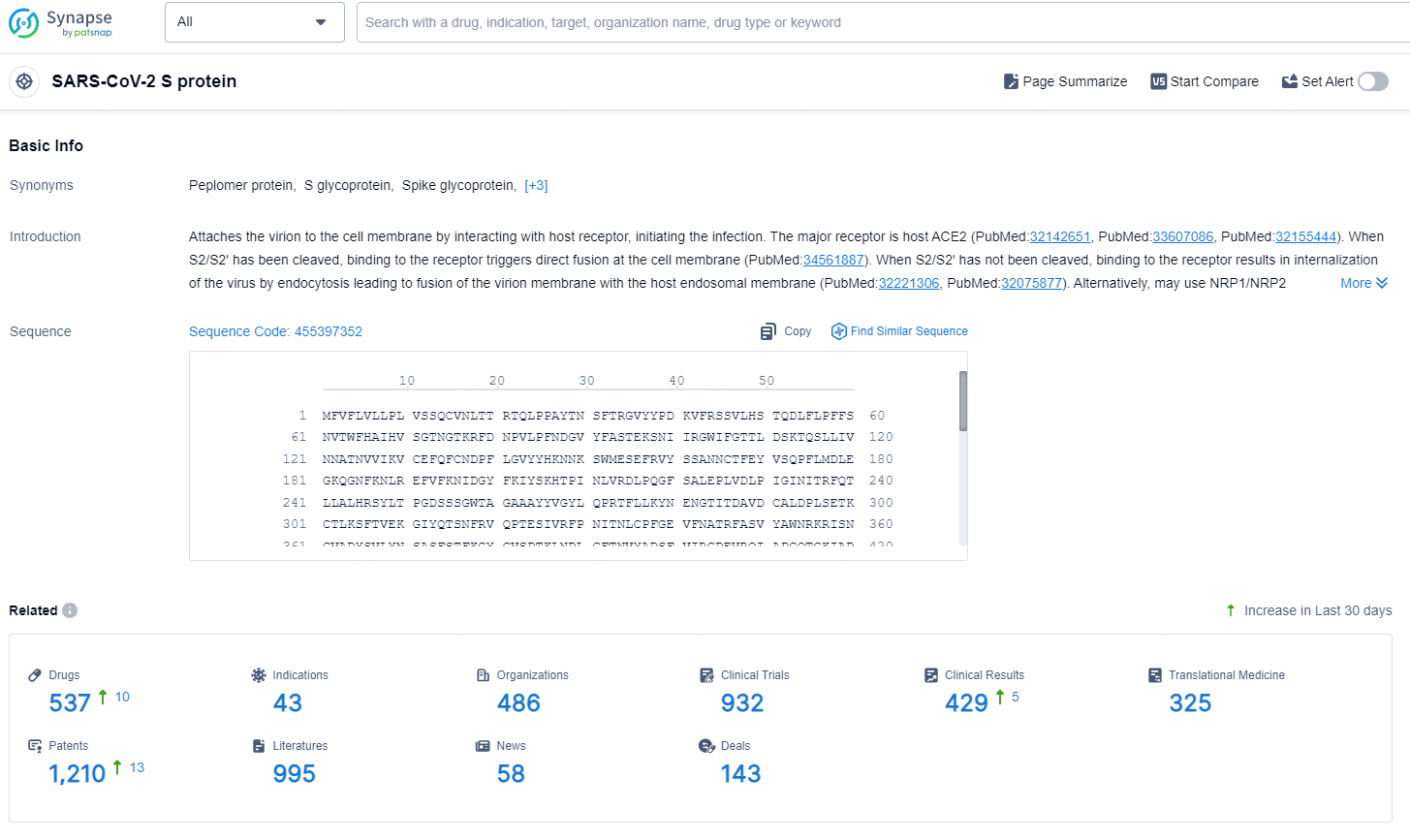
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 26, 2024, there are 537 investigational drugs for the SARS-CoV-2 S targets, including 43 indications, 486 R&D institutions involved, with related clinical trials reaching 932, and as many as 1210 patents.
Tozinameran, also known as famtozinameran, is a prophylactic mRNA vaccine that targets the SARS-CoV-2 S protein, specifically designed to address the COVID-19 disease. It falls under the therapeutic areas of Infectious Diseases and Respiratory Diseases. Developed by BioNTech SE, it has received approval for emergency use authorization in the United States, with the highest phase of approval achieved.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!