How to find the chemical modification of Vutrisiran Sodium?
Vutrisiran Sodium is a novel RNA interference (RNAi) therapeutic developed by Alnylam Pharmaceuticals. Targeting transthyretin (TTR), Vutrisiran Sodium is designed to treat hereditary transthyretin-mediated amyloidosis (hATTR), a rare and life-threatening disease characterized by the accumulation of misfolded TTR protein in various tissues, leading to significant morbidity and mortality. As an RNAi therapeutic, Vutrisiran Sodium represents a significant advancement in the treatment of hATTR, offering a new and effective approach to managing this debilitating condition.
Summary of Research Progress of Vutrisiran Sodium
The research progress of Vutrisiran Sodium has been marked by significant milestones and promising results. Mechanistically, Vutrisiran Sodium works by silencing the messenger RNA (mRNA) that codes for TTR, thereby reducing the production of the TTR protein. This reduction in TTR levels helps prevent the formation of amyloid deposits and can potentially reverse existing deposits, alleviating symptoms and improving patient outcomes. Globally, Vutrisiran Sodium has received regulatory approval in several countries, including the United States, European Union, and Japan, under the brand name Amvuttra. These approvals were based on robust clinical trial data demonstrating the safety and efficacy of the drug.
The global competition in the hATTR market is intense, with several other therapies already available or in late-stage development. Notably, patisiran (Onpattro) and inotersen (Tegsedi), both also developed by Alnylam Pharmaceuticals, are currently approved for the treatment of hATTR. However, Vutrisiran Sodium offers a more convenient dosing schedule, requiring only quarterly subcutaneous injections compared to the monthly intravenous infusions for patisiran and weekly subcutaneous injections for inotersen. This improved dosing regimen enhances patient convenience and compliance, which is crucial for long-term management of chronic conditions like hATTR.
Clinical research on Vutrisiran Sodium has been extensive and rigorous. The pivotal Phase 3 HELIOS-A trial demonstrated that Vutrisiran Sodium significantly reduced serum TTR levels and improved neurological function, quality of life, and other key endpoints compared to placebo. The trial enrolled 164 patients with hATTR polyneuropathy, and the results showed a mean reduction in serum TTR of 81% at six months. Additionally, the safety profile of Vutrisiran Sodium was favorable, with no significant safety concerns identified. These positive findings have solidified Vutrisiran Sodium's position as a leading therapy in the hATTR treatment landscape.
Sequence Information and Characteristics of Vutrisiran Sodium
Vutrisiran Sodium consists of a double-stranded RNA molecule, each strand 21-23 nucleotides long. The sequence of the siRNA is carefully designed to complement the target TTR mRNA, ensuring precise and efficient silencing. The sense strand (passenger strand) and the antisense strand (guide strand) are paired through complementary base pairing. The antisense strand is the active component that hybridizes with the target TTR mRNA, leading to its degradation.
Chemical Modification and Action of Vutrisiran Sodium
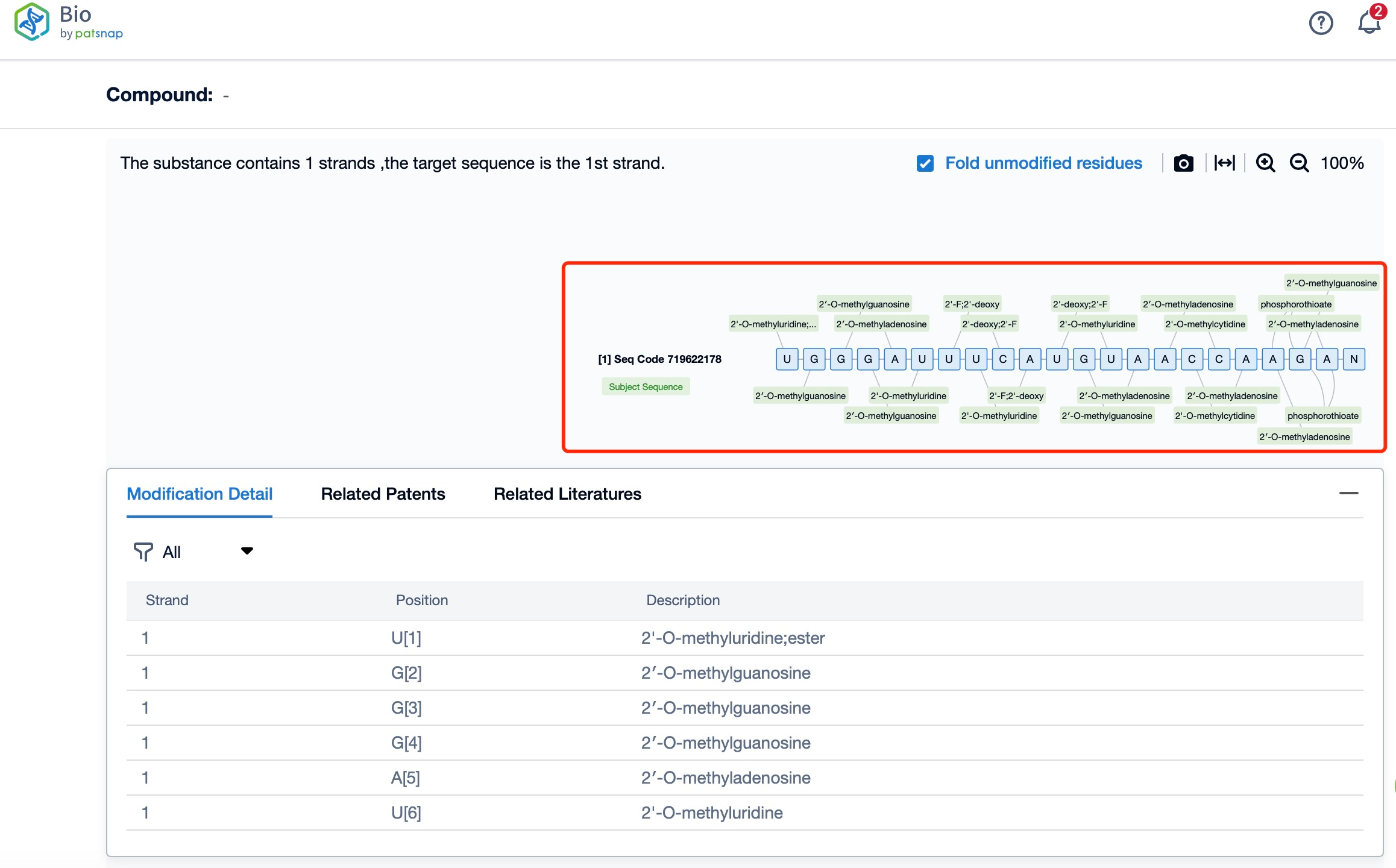
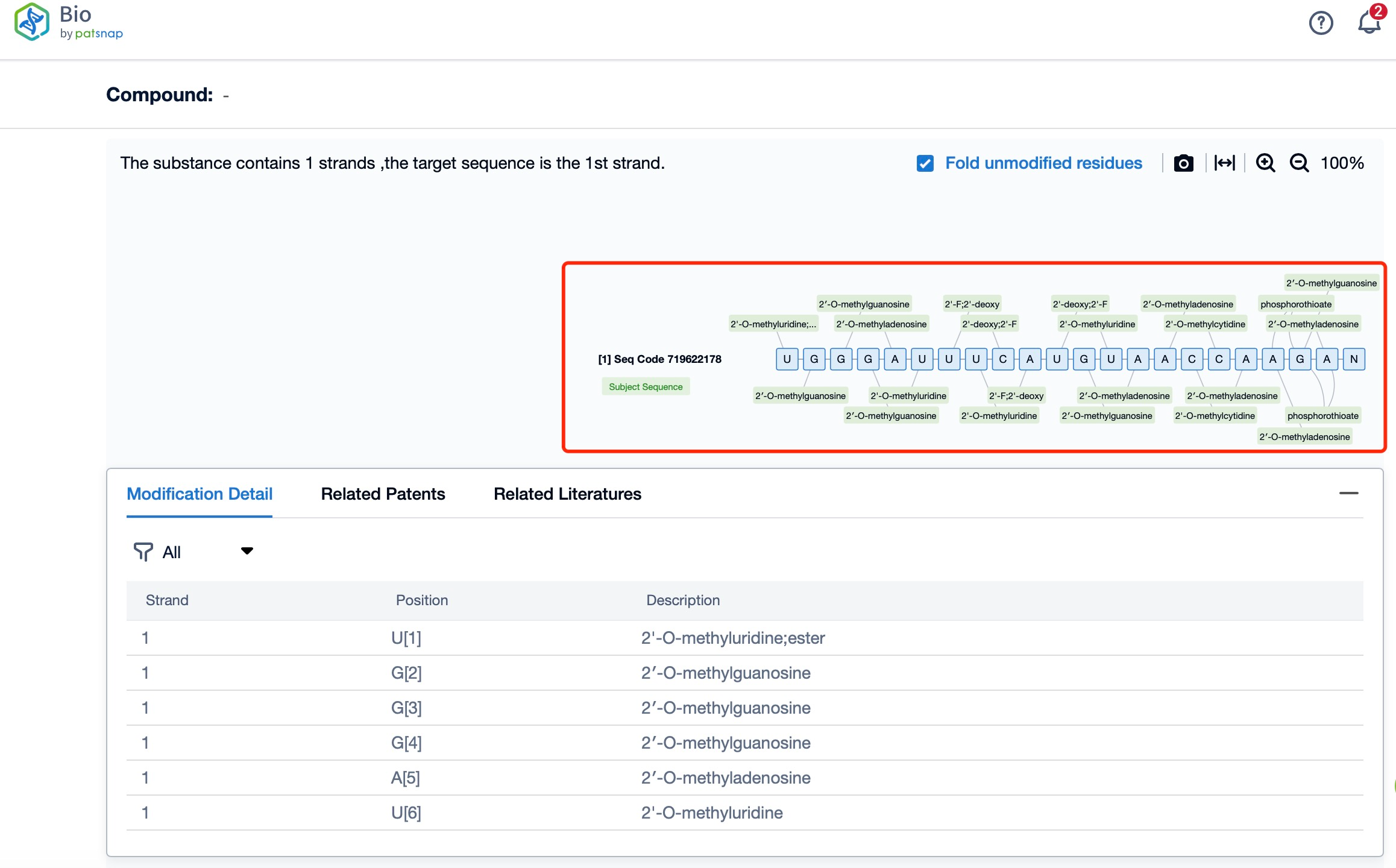
The chemical modifications of Vutrisiran Sodium further enhance its stability and efficacy. The siRNA component of Vutrisiran Sodium undergoes several modifications, including 2'-O-methoxyethyl (MOE) substitutions on the ribose sugar of certain nucleotides. These modifications increase the resistance of the siRNA to degradation by nucleases in the bloodstream and improve its pharmacokinetic properties. Additionally, the GalNAc conjugation improves the solubility and pharmacodynamic properties of the siRNA, allowing for a more stable and effective therapeutic molecule.
The action of Vutrisiran Sodium is mediated through the RNAi pathway. Once inside the hepatocytes, the GalNAc-siRNA conjugate is processed by the RNA-induced silencing complex (RISC). The passenger strand of the siRNA is degraded, while the guide strand remains bound to the RISC. The guide strand then hybridizes with the complementary TTR mRNA, leading to its cleavage and degradation. This process effectively silences the TTR gene, reducing the production of TTR protein and preventing the formation of amyloid deposits. The reduction in TTR levels is sustained over time, providing long-lasting therapeutic benefits.
Summary and Prospect
In summary, Vutrisiran Sodium represents a significant advancement in the treatment of hereditary transthyretin-mediated amyloidosis (hATTR). Its unique mechanism of action, involving the targeted silencing of TTR mRNA, offers a novel and effective approach to managing this debilitating condition. The drug's favorable safety profile, combined with its convenient dosing schedule, makes it an attractive option for patients with hATTR. With regulatory approvals in multiple regions and strong clinical trial data, Vutrisiran Sodium is poised to play a crucial role in the future of hATTR treatment. Ongoing research and development efforts aim to further optimize the drug's therapeutic potential and explore its use in other related diseases, opening up new possibilities for patients suffering from rare and complex disorders.
How to find the chemical modification of all siRNAs?
In Patsnap Bio, you can find the sequence and latest research and development advances of all siRNAs.
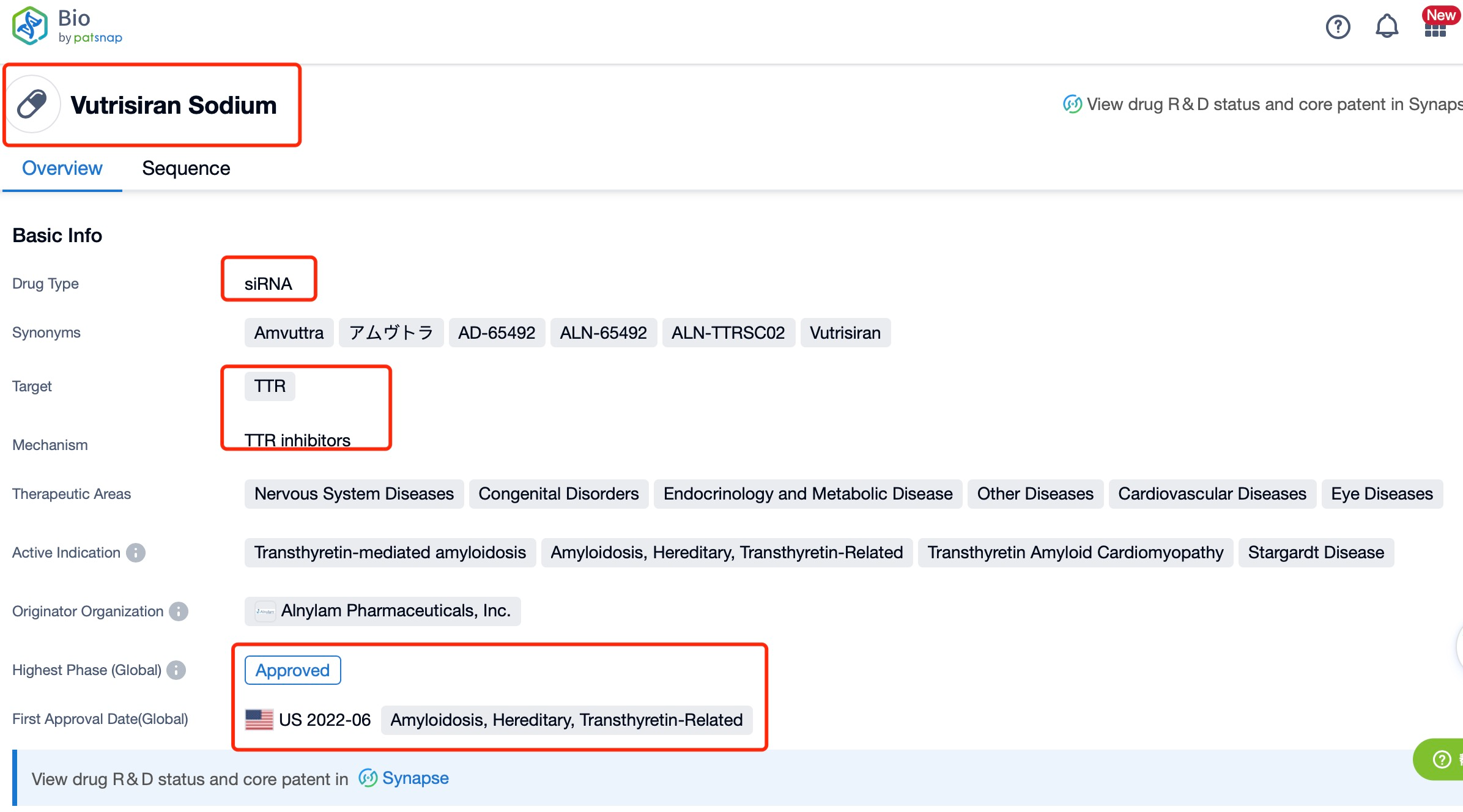
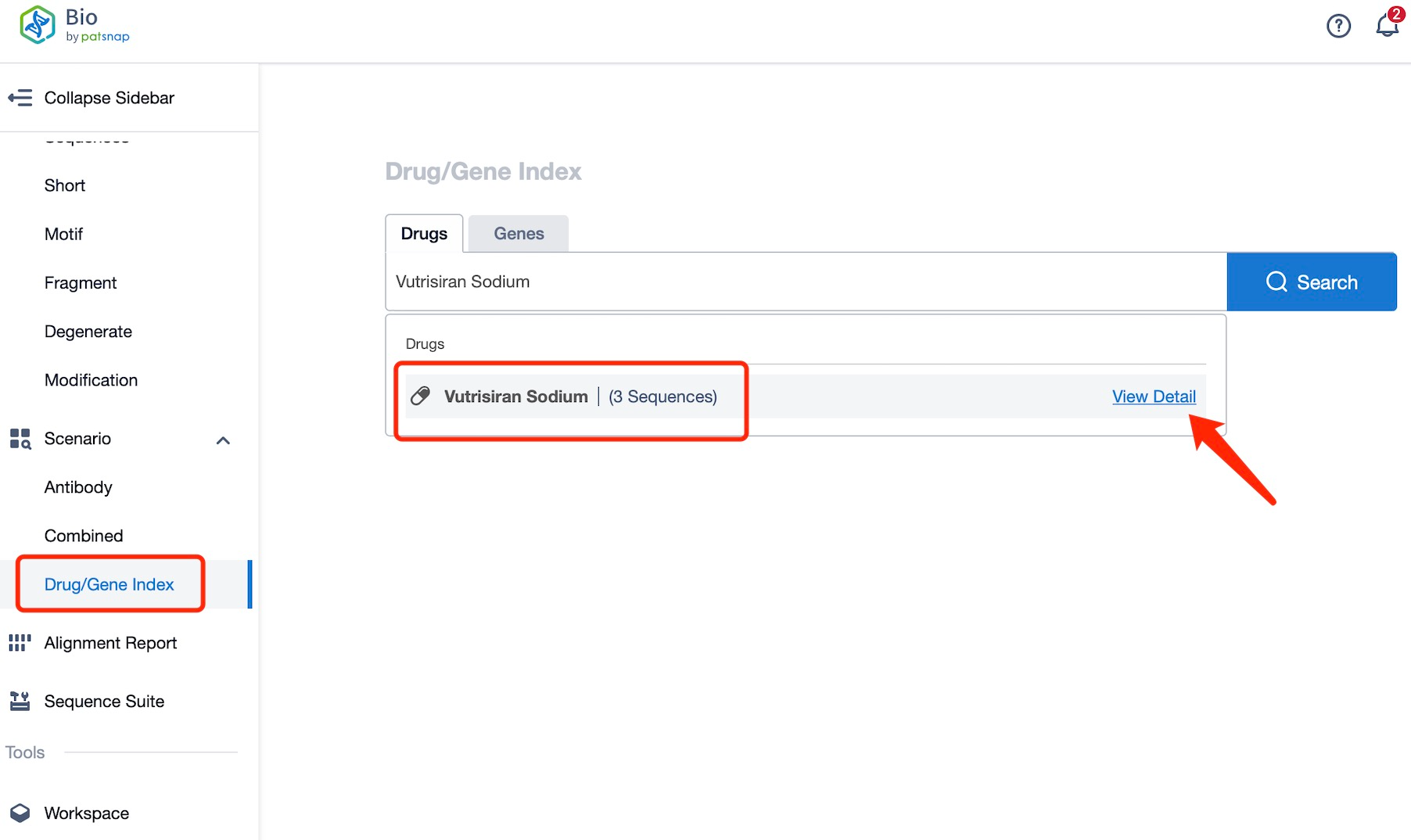
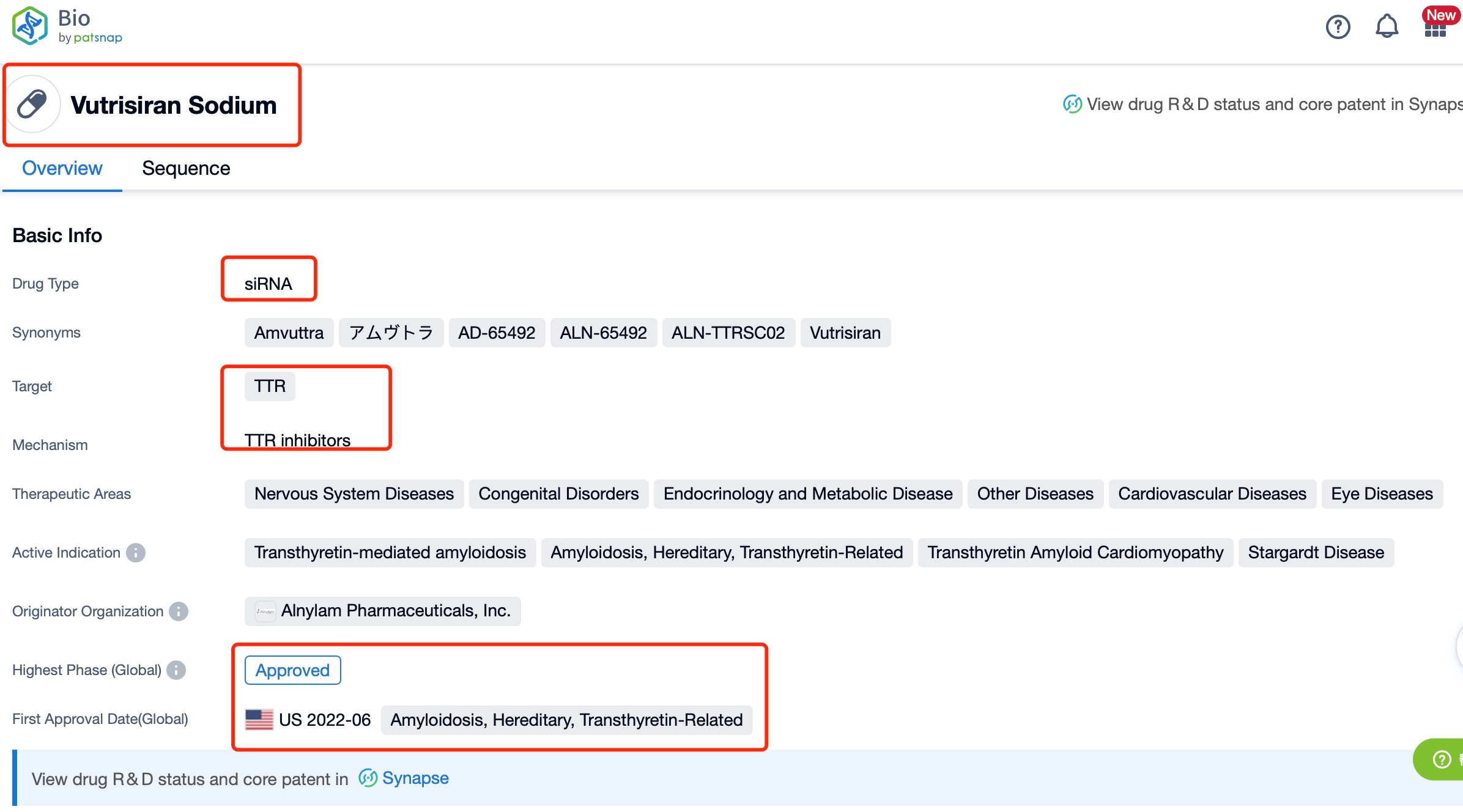
Taking Vutrisiran Sodium as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' Vutrisiran Sodium ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of Vutrisiran Sodium.
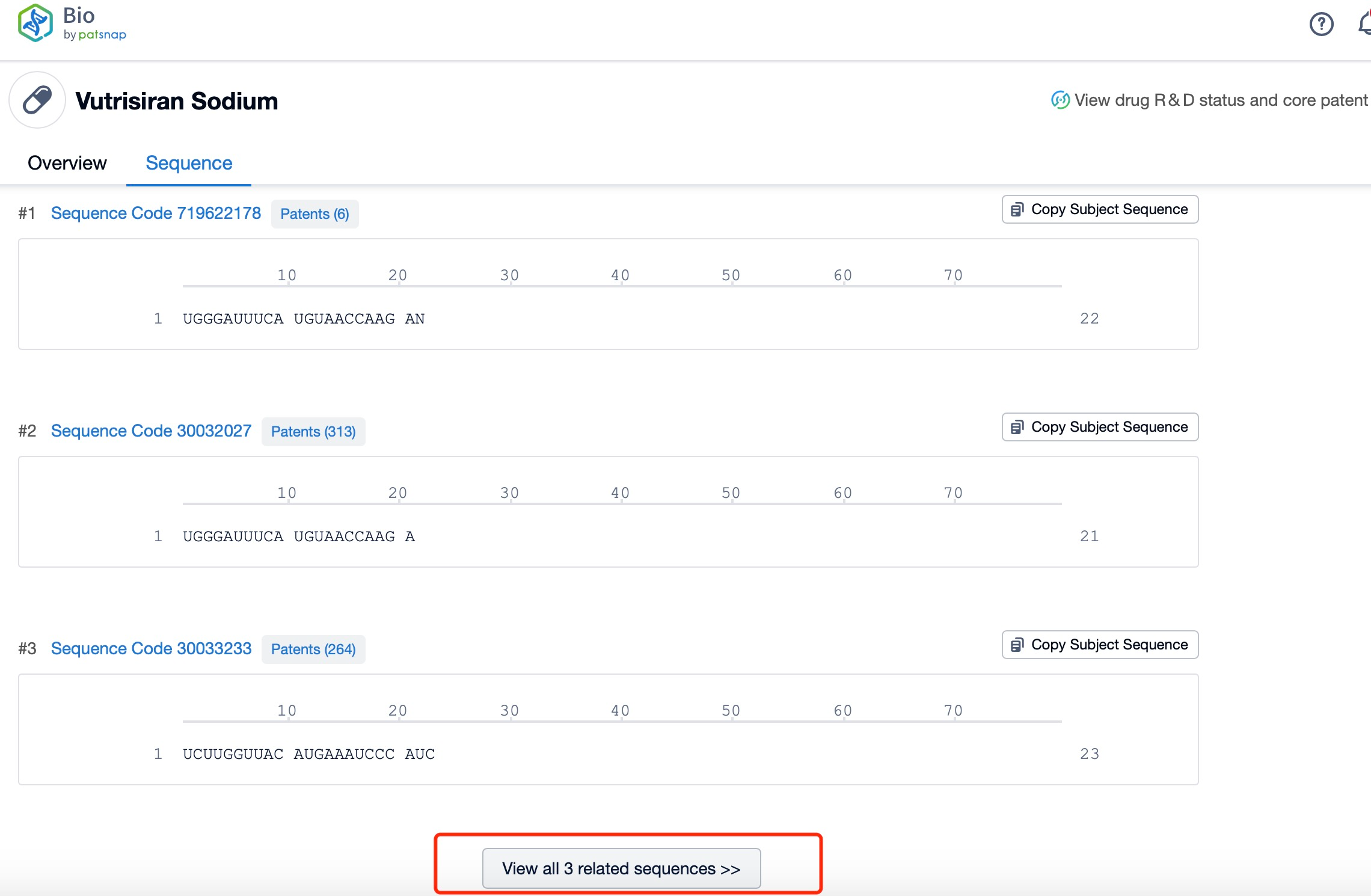
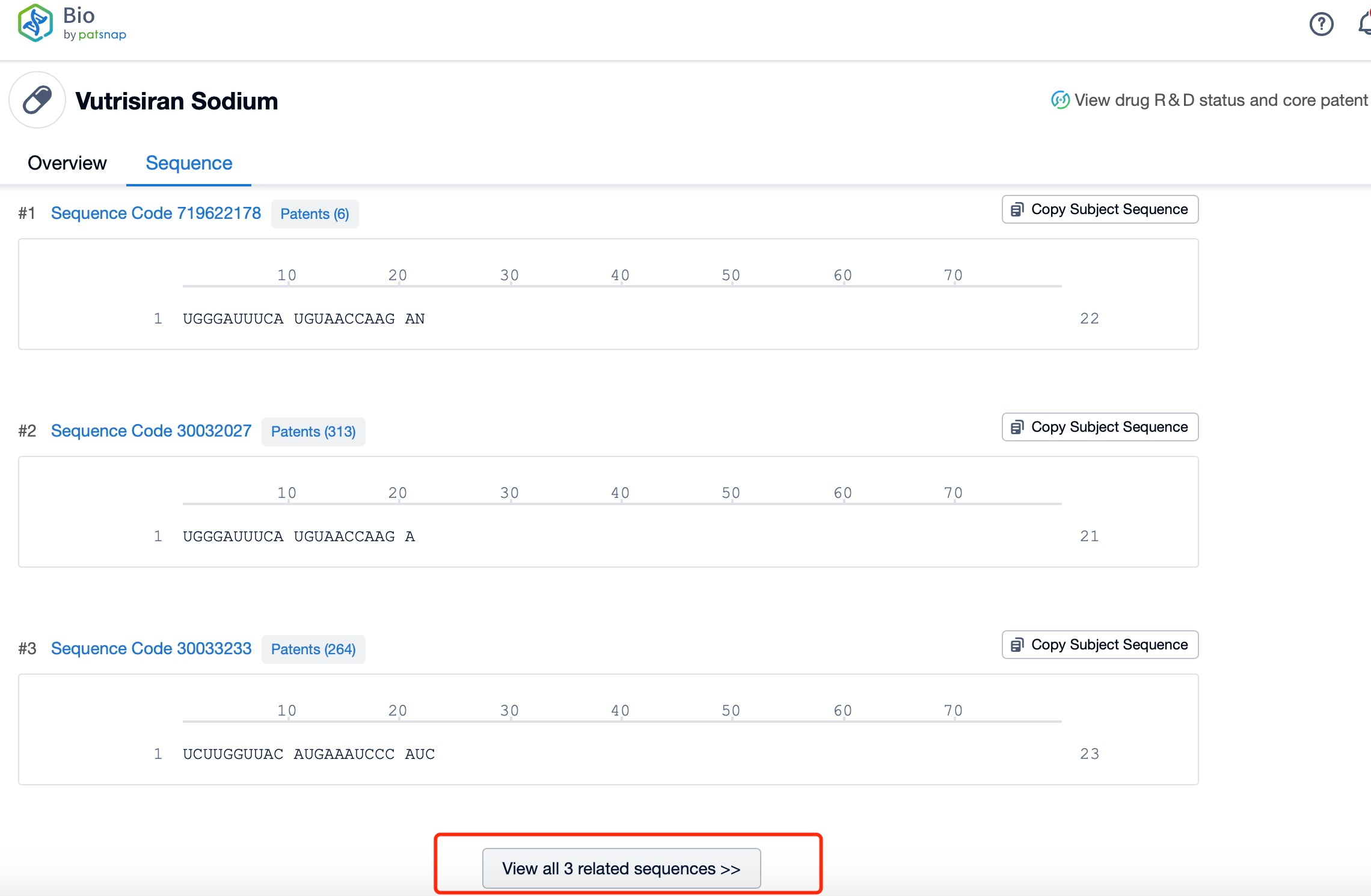
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
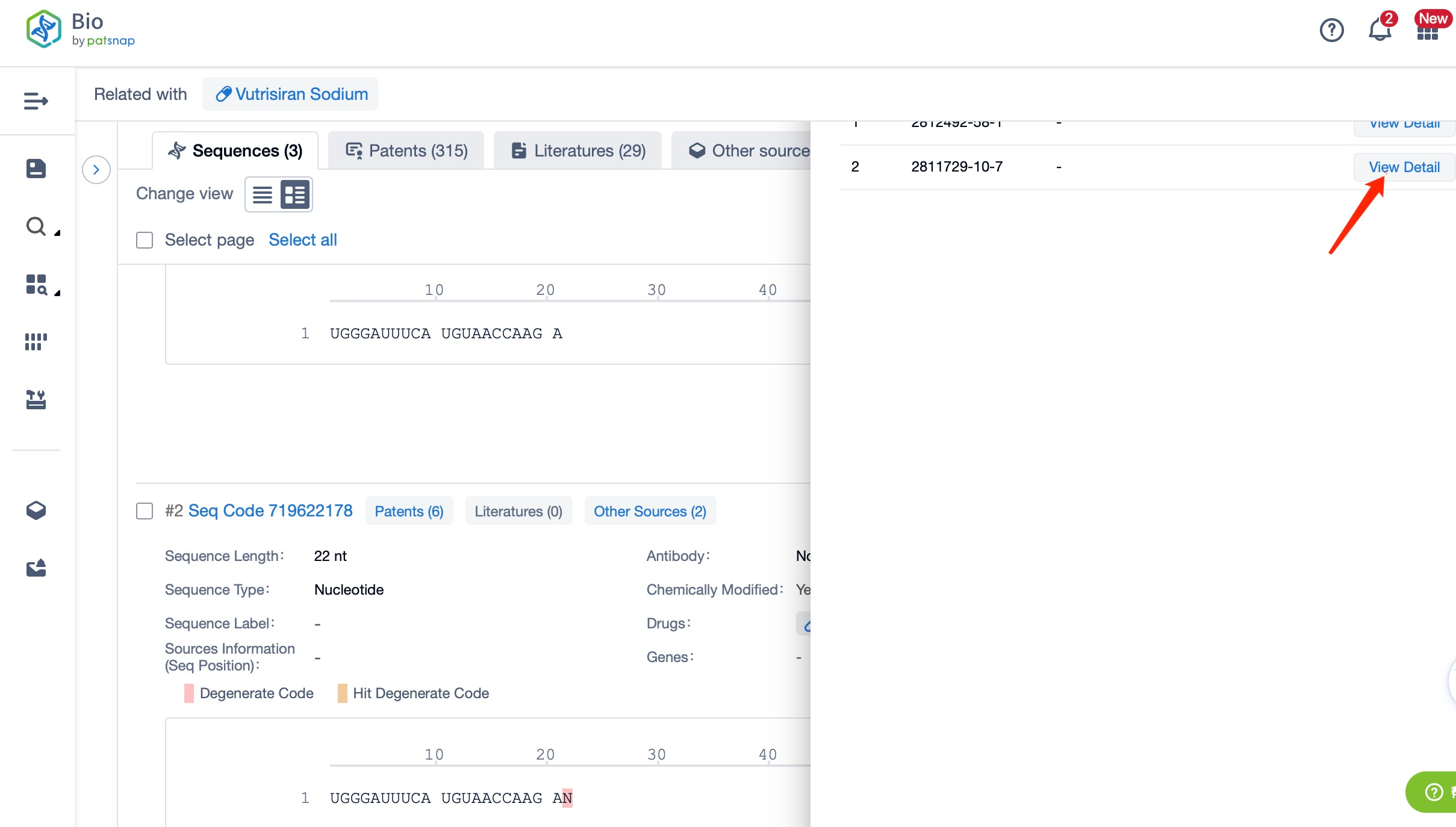
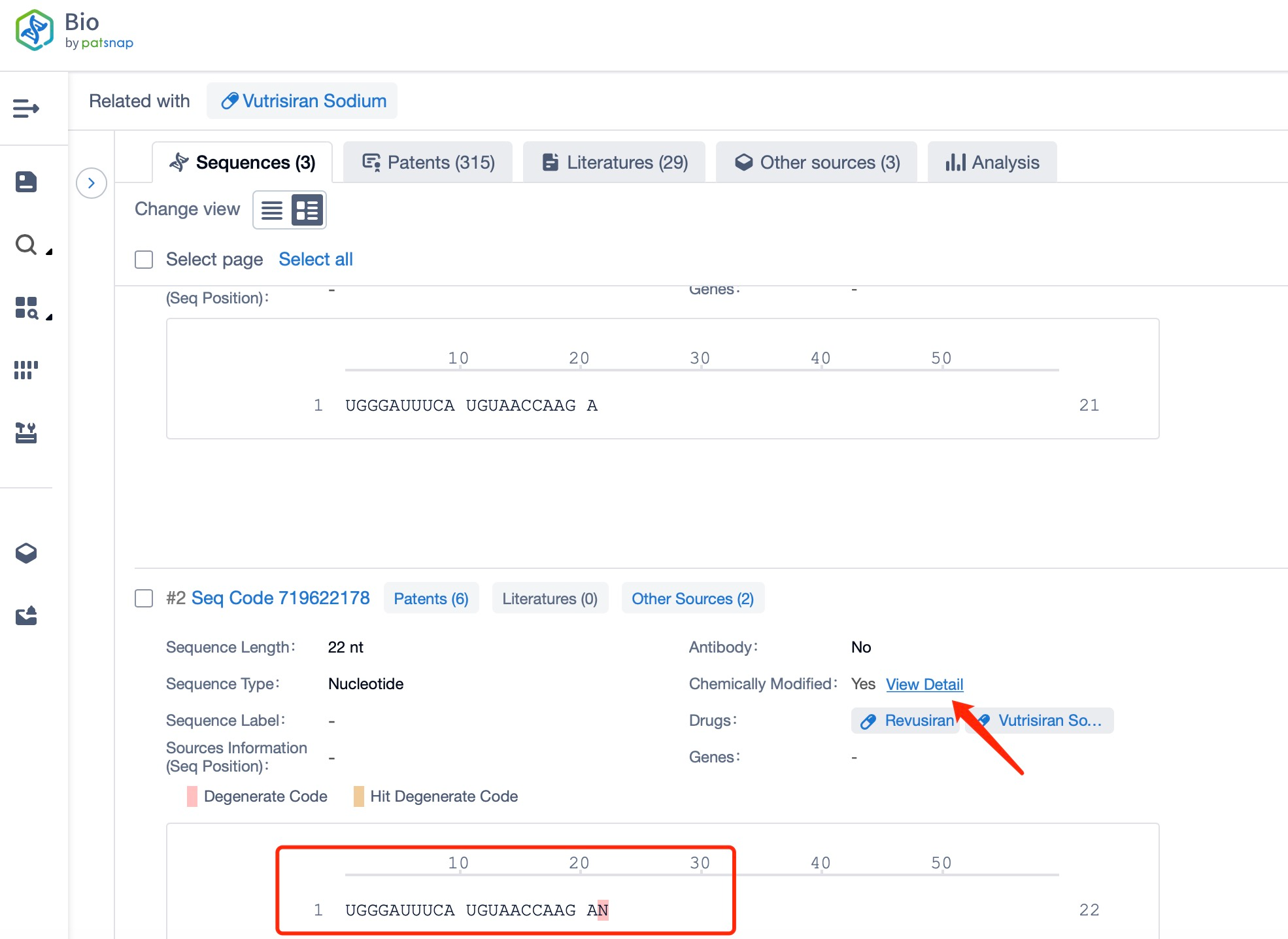
Clicking on the sequence name will provide you with all the basic information of that sequence.
Additionally, a visual diagram of the sequence's chemical modifications is available for immediate access.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.