Advancements in CMTX-101 Antibody Treatment for Cystic Fibrosis Infections Revealed by Clarametyx Biosciences
Clarametyx Biosciences, Inc., a biotechnology company concentrating on unique biologic therapies designed to enhance immune responses against serious infections associated with biofilms, has reported the successful conclusion of the first Phase 1b part of its clinical trial. This investigation is evaluating the novel immune-stimulating antibody treatment for addressing pulmonary infections connected to cystic fibrosis (CF). The company is now prepared to initiate the Phase 2a portion of the study.
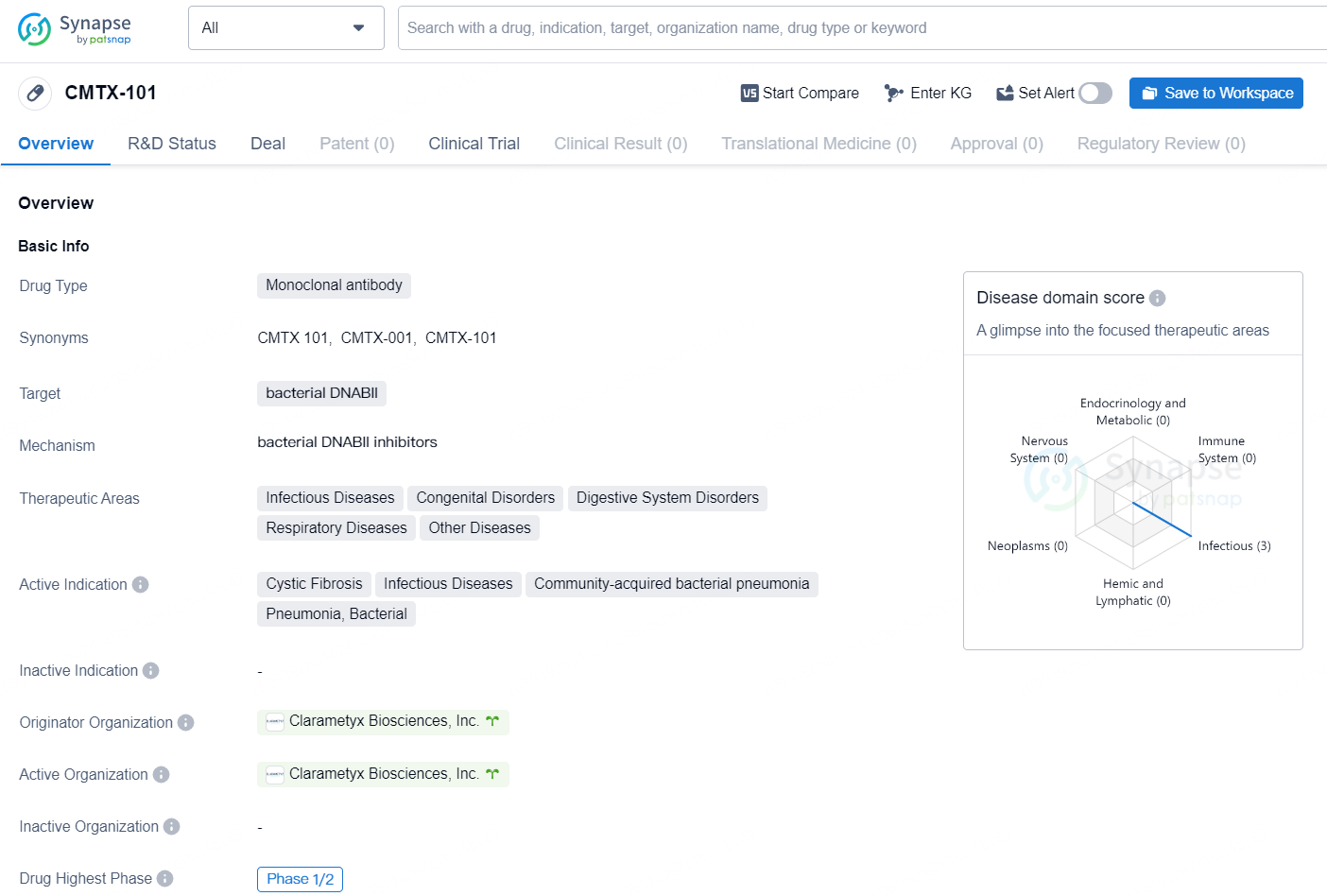
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Cystic fibrosis (CF) patients are at a substantial risk of chronic and recurrent lung infections, underscoring the need for treatment options that improve bacterial clearance while reducing dependence on antibiotics," remarked Jerry Nick, MD, a Professor in the Division of Pulmonary, Critical Care, and Sleep Medicine at the National Jewish Health Department of Medicine and the principal investigator of the study. "We are encouraged by initial results from the Phase 1b segment of the research, which showed no safety issues with CMTX-101. Should additional clinical assessments verify its capacity to interfere with bacterial defenses and enhance reactions to antibiotics and natural immunity, CMTX-101 could represent a groundbreaking treatment method for individuals with CF."
This randomized, double-blind, placebo-controlled trial is designed to investigate the safety and tolerability of CMTX-101, as well as its pharmacokinetics, immunogenicity, and effectiveness in reducing pulmonary P. aeruginosa levels, among other exploratory outcomes, when administered alongside standard antibiotic therapies for CF patients. Following the completion of the Phase 1b stage and a review of safety data by independent committees, the ongoing Phase 2a aims to enroll up to 41 adult CF patients to further evaluate the safety and tolerability of CMTX-101. The organization plans to release initial findings of this study by 2025. For more information on the research and participating sites, visit ClinicalTrials.gov (http://www.clinicaltrials.gov/) with the identifier NCT06159725.
The development of technologies targeting CF-related infections is consistent with the company's strategic aim to address persistent and chronic infections associated with various bacterial pathogens. Recently, the firm completed a preliminary clinical trial assessing CMTX-101 for community-acquired bacterial pneumonia, yielding important data on safety, tolerability, pharmacokinetics, immunogenicity, and exploring efficacy outcomes. Notably, the trial found no major safety concerns, a lower incidence of anti-drug antibodies, and an absence of neutralizing antibodies. Clarametyx is scheduled to present positive preliminary findings from this study on October 18th at IDWeek in Los Angeles.
"We recognize that recurrent infections present a significant challenge for cystic fibrosis patients and their families. We are convinced that an innovative strategy to support the immune system of patients, alongside current antibiotics, can effectively tackle these infections, reducing the burden for patients, promoting better antibiotic practices, and possibly addressing resistance issues," said David Richards, Chief Executive Officer. "With encouraging preliminary data from the Phase 1b segment of our CF study, we are excited to advance to the next development stage to demonstrate the substantial potential of our technology in transforming treatment strategies for chronic and persistent infections."
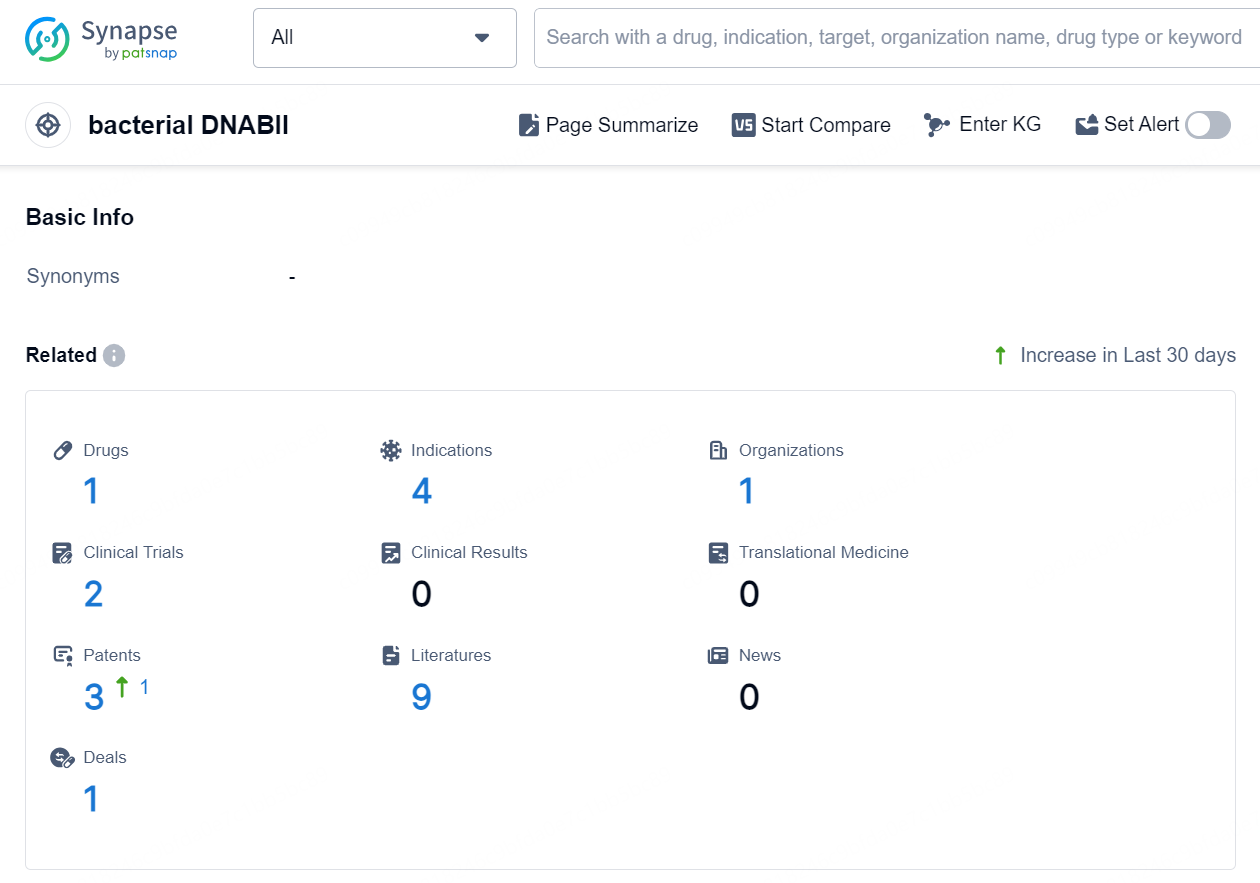
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 21, 2024, there are 1 investigational drug for the bacterial DNABII target, including 4 indications, 1 R&D institution involved, with related clinical trials reaching 2, and as many as 3 patents.
CMTX-101 is a monoclonal antibody drug developed by Clarametyx Biosciences, Inc. The drug targets bacterial DNABII and is being developed for the treatment of various therapeutic areas including Infectious Diseases, Congenital Disorders, Digestive System Disorders, Respiratory Diseases, and Other Diseases. The drug is currently in the highest phase of Phase 1/2 globally.