First Patient Dosed in Bright Peak's BPT567 Phase 1/2a Trial for PD1-IL18 Immunoconjugate
Bright Peak Therapeutics, a biotechnology firm in the clinical development phase that specializes in discovering and advancing multifunctional immunotherapies for cancer treatment, has revealed that the initial patient has received their dose in the Phase 1/2a trial assessing BPT567, a candidate bifunctional PD1-IL18 immunoconjugate.
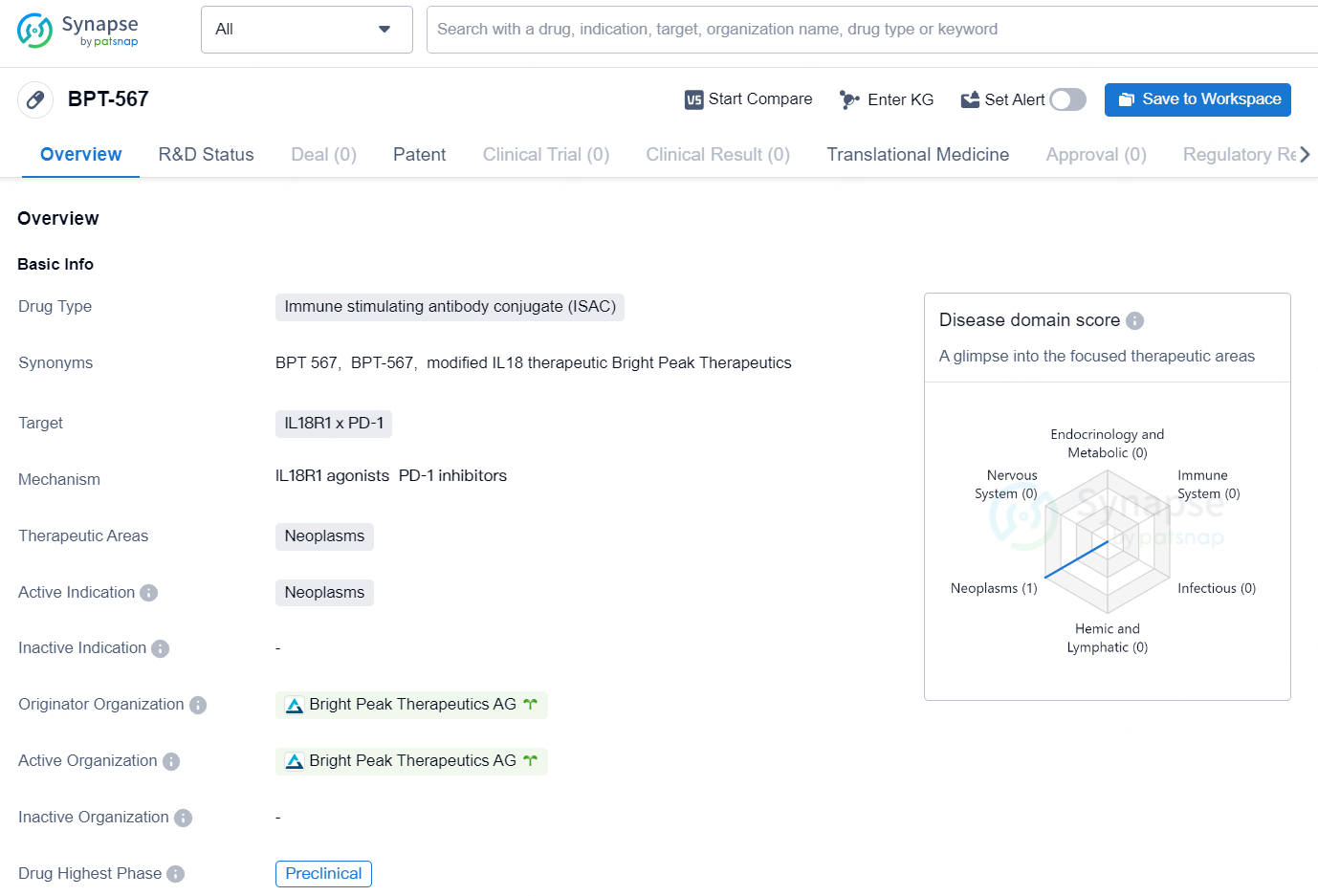
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
BPT567 has been engineered to integrate two significant immuno-stimulatory action mechanisms within a singular compound. This includes the coordinated blockade of the PD-1/PD-L1 checkpoint combined with the specific delivery of IL-18 to T cells located in the tumor microenvironment (TME). IL-18 serves as a principal regulator for both innate and adaptive immunity, acting as a catalyst for the host immune response against cancer. Preclinical findings indicate that BPT567 facilitates substantial, synergistic anti-tumor effects that outperform the use of PD-1 blockade alone, demonstrating efficacy in both PD-1-sensitive and PD-1-resistant tumor models.
Although PD-1 inhibitors have transformed cancer immunotherapy, Bright Peak is actively exploring the potential of BPT567’s multifunctional properties. They aim to determine whether it can achieve greater efficacy compared to PD-1/PD-L1 blockade in conditions where checkpoint inhibitors are currently approved, as well as exhibit effectiveness in scenarios where checkpoint inhibitors have failed or in patients who have experienced progression or recurrence following prior checkpoint therapy. These areas highlight a significant unmet need among cancer patients.
“The initiation of dosing for the first participant in this trial is a major and exciting milestone in our pursuit of offering advanced solid tumor patients a potentially transformative PD-1-based treatment alternative,” stated Bright Peak's CEO, Fredrik Wiklund.
Jon Wigginton, M.D., President of Research and Development at Bright Peak, remarked, “Encouraging results from preclinical studies of BPT567 motivate us to delve deeper into its dual mechanisms. We believe it holds the capability to provide meaningful anti-tumor efficacy, particularly for patients who have not responded to traditional PD-1 inhibitors, and in new indications not currently encompassed by existing PD-1 therapies. We anticipate progressing in this critical Phase 1/2a study in partnership with participating patients and leading centers focused on immuno-oncology.”
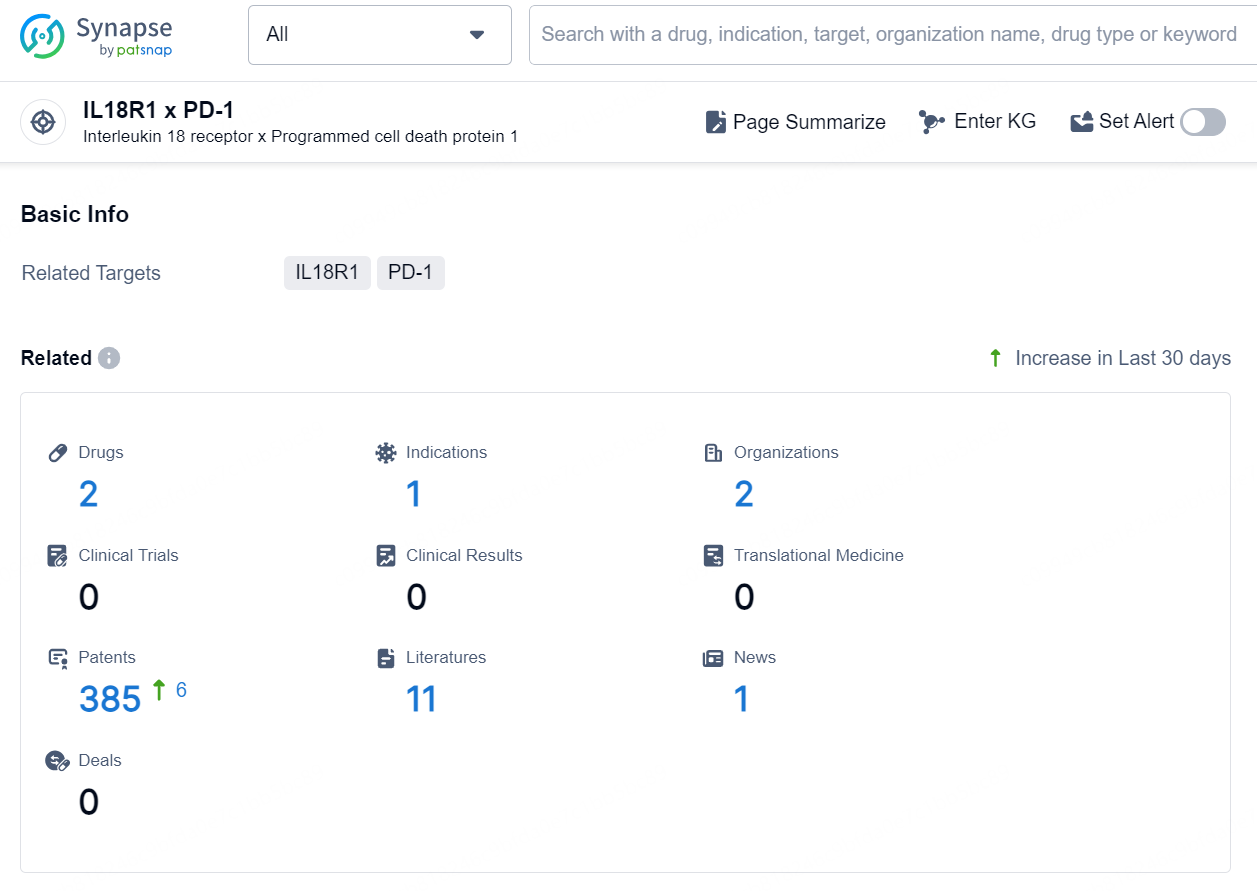
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 21, 2024, there are 2 investigational drugs for the IL18R1 x PD-1 target, including 1 indication, 2 R&D institutions involved, and as many as 385 patents.
The drug BPT-567 is classified as an Immune Stimulating Antibody Conjugate (ISAC) and targets IL18R1 x PD-1. It is intended for use in the therapeutic area of neoplasms, with an active indication for the treatment of neoplasms. The drug is currently in the preclinical phase and is being developed by Bright Peak Therapeutics AG.