Takeda Reveals Promising Initial Findings from Phase 2b Trial Examining TAK-279, aimed at treatment of Active Psoriatic Arthritis
Takeda has recently publicized encouraging preliminary findings from its multi-dose Phase 2b trial, which was conducted using randomization, a double-blind method, and a placebo control. This investigation assessed the efficiency of TAK-279, an oral allosteric tyrosine kinase (TYK2) inhibitor with advanced selectivity, in treating patients diagnosed with active psoriatic arthritis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The research reached its primary goal as a larger percentage of participants given TAK-279 once daily saw a minimum of a 20 percent easing in disease signs and manifestations at week 12, relative to the placebo group. This highlights the potential of TAK-279 to serve as a highly selective oral treatment option for patients with psoriatic arthritis. The safety and tolerance profile of TAK-279 in the Phase 2b experiment had a concurrence with prior clinical trials of the compound. Examination of the data continues, and Takeda anticipates revealing the medical outcomes at an upcoming medical meeting.
"Psoriatic arthritis places a significant weight on affected individuals and there is a clear need for more therapeutic alternatives that offer effectiveness, safety, tolerance, and convenience," said Andy Plump, R&D President at Takeda. "We postulate that heightened selectivity, as demonstrated with TAK-279, could allow for robust TYK2 inhibition while likely steering clear of toxicities linked to JAK inhibition. We eagerly anticipate unveiling the findings shortly and investigating the possible benefits of TAK-279 in further clinical research."
Psoriatic arthritis is a chronic condition driven by the immune system, marked by inflammation-triggered joint discomfort, rigidity, and swelling. The illness impacts nearly 10 million individuals worldwide. The chronic inflammation seen in psoriatic arthritis may lead to permanent joint damage if not properly managed, and the progressive nature of the disease aligns with severe physical disability and significant mental health concerns such as anxiety and depression.
Based on the Phase 2b results, Takeda aims to kickstart a Phase 3 trial of TAK-279 in psoriatic arthritis. A Phase 3 assessment of TAK-279 in plaque psoriasis is slated for initiation in FY2023. Takeda is also planning to evaluate TAK-279's potential in other immune-mediated inflammations such as systemic lupus erythematosus, Crohn’s disease, ulcerative colitis.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
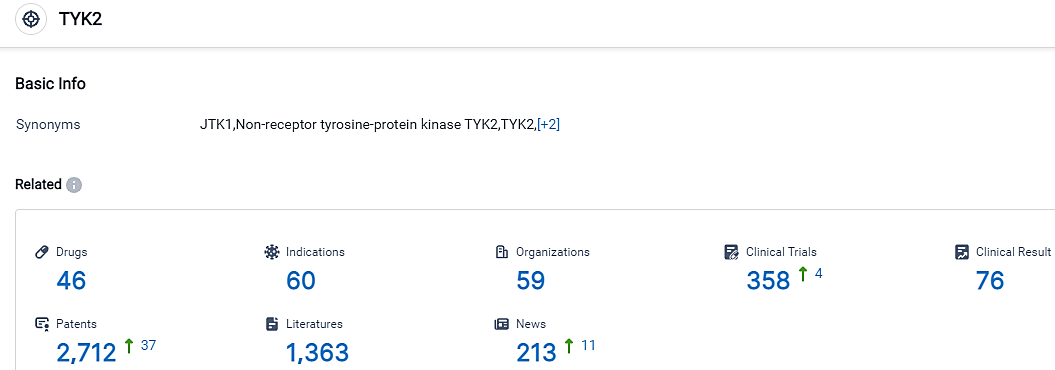
According to the data provided by the Synapse Database, As of September 17, 2023, there are 46 investigational drugs for the TYK2 target, including 60 applicable indications,59 R&D institutions involved, with related clinical trials reaching 358,and as many as 2712 patents.
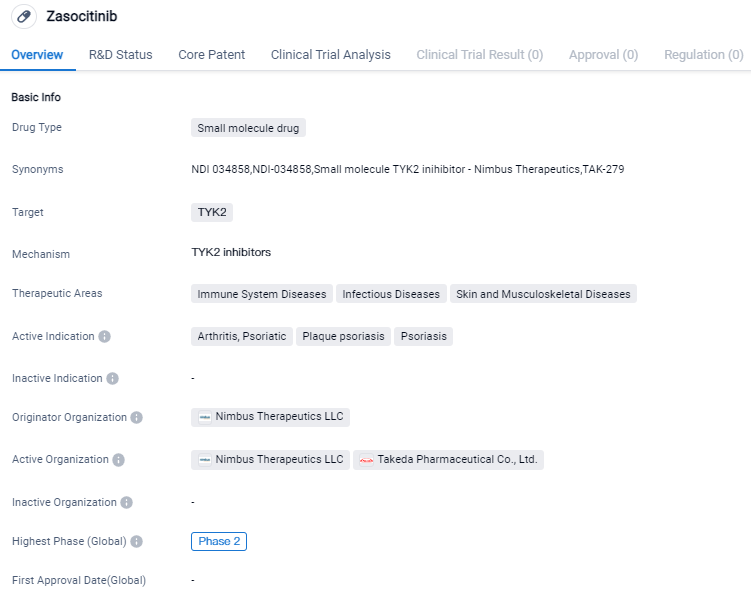
Zasocitinib's development as a small molecule drug targeting TYK2 for the treatment of immune system diseases, infectious diseases, and skin and musculoskeletal diseases is currently in Phase 2, with Nimbus Therapeutics LLC as the originator organization. Zasocitinib is an investigational compound that has not been approved for use by any regulatory authority. Further research and clinical trials will be necessary to determine the drug's safety and efficacy in treating the indicated conditions.