Another Potential Stock after PD1 - LAG3 Inhibitors
The Lymphocyte-activation gene-3 (LAG-3) is located on human chromosome 12 (20p13.3), includes 8 exons, and its corresponding cDNA encodes a membrane protein containing 498 amino acids. It is an immune checkpoint protein expressed on the surface of effector T cells and regulatory T cells (Tregs), and can modulate signal pathways of T lymphocytes and antigen-presenting cells (APCs). LAG-3 plays a vital role in adaptive immune responses.
Similar to PD-1 and CTLA-4, LAG-3 is not expressed on naive T cells but can be induced on CD4+ and CD8+T cells upon antigen stimulation. The inhibitory function of LAG-3 is closely related to its expression level on the cell surface, hence the regulation of LAG-3 expression is critical. Long-term exposure to antigens due to viral, bacterial, and parasitic infections can lead to the prolonged high-level expression of LAG-3 and other inhibitory co-receptors on CD4+ and CD8+T cells.
These T cells lose their potent effector functions, referred to as exhausted T cells, resulting in decreased tumor killing ability and response rate, and upregulated immunosuppressive function of Tregs.
Studies show that inhibition of LAG-3 can restore cytotoxic activity of T cells, reduce the immunity suppressing function of regulatory T cells, hence enhancing anti-tumor effects. Research displays dual inhibitory effects when simultaneously blocking the activity of LAG-3 and anti-PD-1 or PD-L1 in tumor cells, including inhibition of Treg activity, promotion of DC maturation, and rescue of function-abnormal CD4+/CD8+T cells. LAG-3 has now become a new target for tumor immunotherapy following CTLA-4, PD-1, and PD-L1.
LAG3 Competitive Landscape
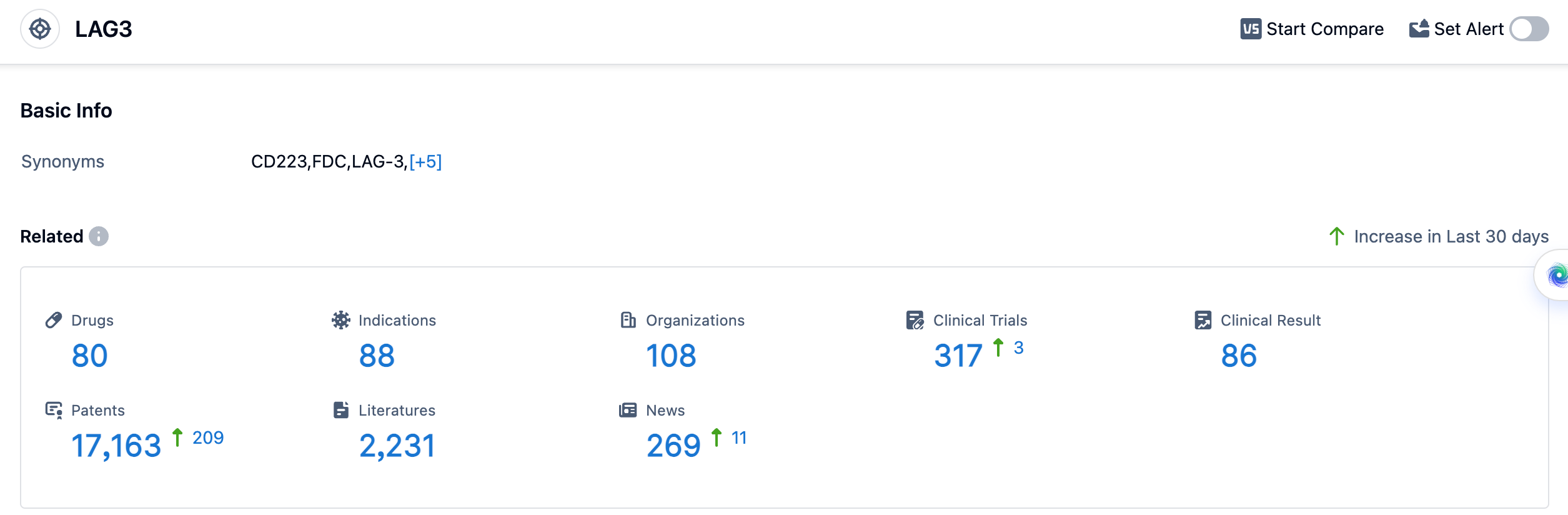
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 80 LAG3 drugs worldwide, from 108 organizations, covering 88 indications, and conducting 317 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Understanding the role of LAG3 in the human body is essential for developing novel immunotherapies and targeted therapies for various diseases, including cancer and autoimmune disorders.
The analysis of the target LAG3 reveals a competitive landscape with multiple companies actively involved in the R&D of drugs. The indications for LAG3-targeting drugs cover a wide range of cancers, indicating the potential for LAG3 as a therapeutic target in oncology. Monoclonal antibodies, bispecific antibodies, and small molecule drugs are the most rapidly progressing drug types. The presence of biosimilars suggests intense competition in the market. Various countries/locations, including China, are actively developing LAG3-targeting drugs, indicating global interest in this target. Overall, the target LAG3 shows promise in the pharmaceutical industry, and further research and development efforts are expected to drive its future development.
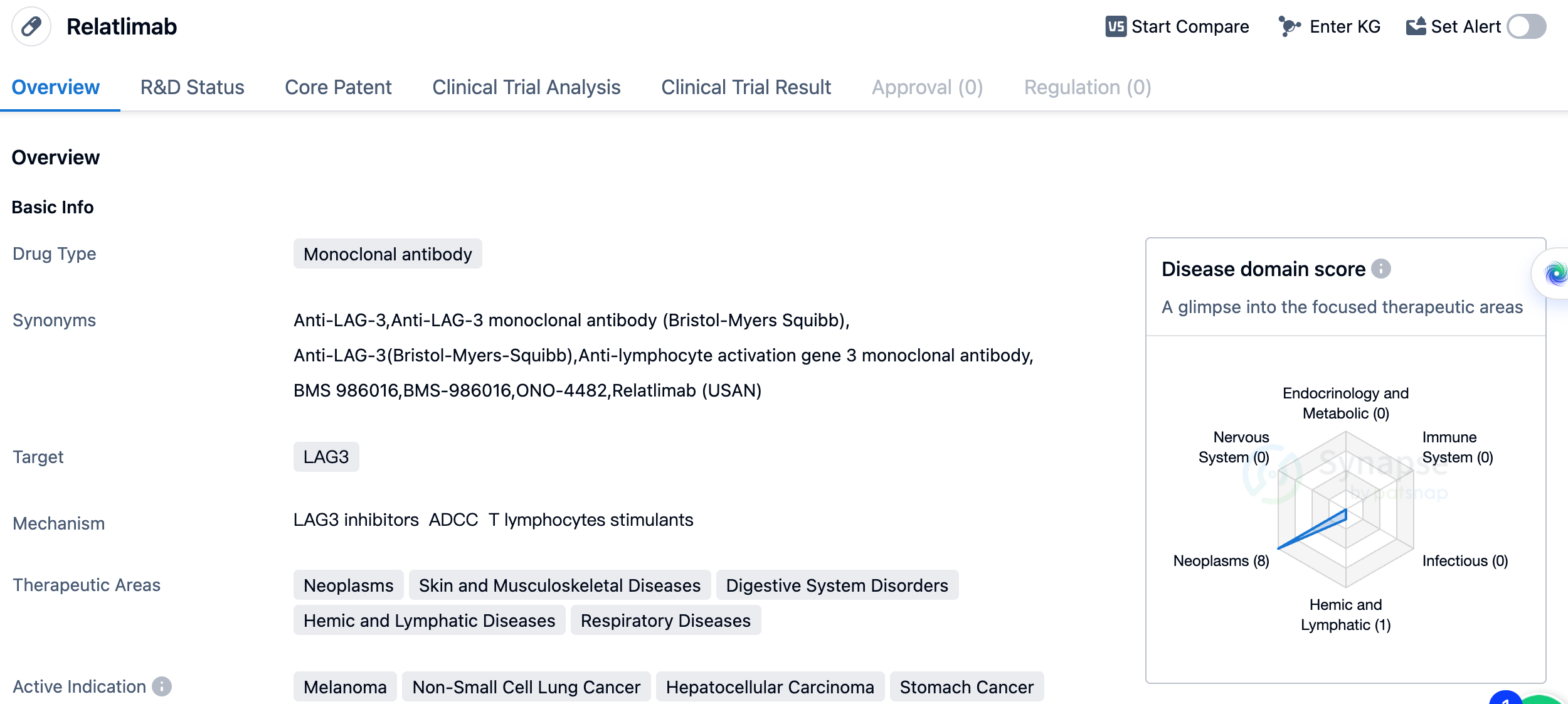
The first LAG3 inhibitor: Relatlimab
Relatlimab is the world's first LAG3 inhibitor, a type of IgG4 monoclonal antibody. In March of this year, it was approved in the United States as part of the combination drug Opdualag®, which was developed by Bristol-Myers Squibb for the first-line treatment of metastatic or inoperable melanoma. Clinically, the median progression-free survival period with Opdualag® is 10.1 months, compared to 4.6 months when Nivolumab (Opdivo®) is used alone. Additionally, the toxicity of this combination is comparable to that of Opdivo® and is better than another combination of Nivolumab and Ipilimumab (anti-CTLA-4 monoclonal antibody), which is used to treat melanoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The therapeutic areas that Relatlimab aims to address are diverse and include neoplasms (abnormal growth of cells), skin and musculoskeletal diseases, digestive system disorders, hemic and lymphatic diseases, and respiratory diseases. This indicates that the drug has the potential to be used in a wide range of medical conditions. By targeting LAG3, the drug aims to enhance the body's immune system to fight against cancer cells and other diseases.
In summary, Relatlimab is a monoclonal antibody drug developed by Bristol Myers Squibb Co. that targets LAG3. It is currently in Phase 3 of clinical trials globally and Phase 2 in China. The drug has a broad range of therapeutic areas and active indications, primarily focusing on various types of cancers. Its development by a reputable pharmaceutical company and its advancement to later stages of clinical trials indicate its potential as a promising treatment option in the field of biomedicine.
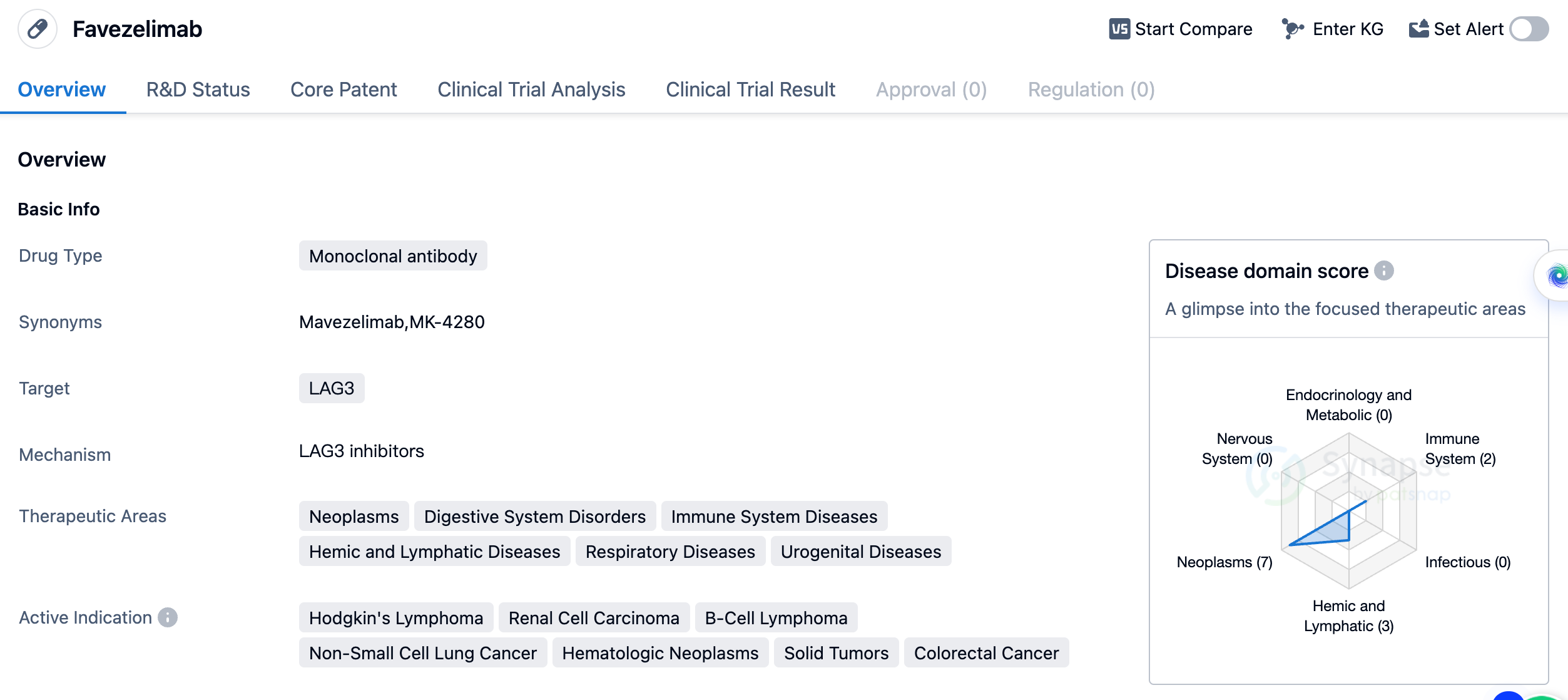
Favezelimab
Favezelimab is a monoclonal antibody drug that targets LAG3, a protein involved in immune regulation. It is being developed by Merck Sharp & Dohme Corp. and has reached the highest phase of clinical development, Phase 3, globally. In China, it has reached Phase 2. The therapeutic areas that Favezelimab aims to address include neoplasms (abnormal growth of cells), digestive system disorders, immune system diseases, hemic and lymphatic diseases, respiratory diseases, and urogenital diseases. This indicates a broad potential for the drug to be used in various medical conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a monoclonal antibody, Favezelimab is designed to specifically target LAG3 and modulate the immune response. This mechanism of action suggests that the drug may have potential in enhancing the body's immune system to fight against cancer cells or other diseases related to immune dysfunction. The fact that Favezelimab has reached Phase 3 in global development indicates that it has shown promising results in earlier stages of clinical trials. Phase 3 trials involve a larger number of participants and are conducted to further evaluate the drug's safety and efficacy. This suggests that Favezelimab may be closer to potential regulatory approval and commercialization. In China, Favezelimab has reached Phase 2, which means that it is still undergoing clinical trials to assess its safety and effectiveness in a Chinese population.
In summary, Favezelimab is a monoclonal antibody drug targeting LAG3, being developed by Merck Sharp & Dohme Corp. It has reached Phase 3 globally and Phase 2 in China. The drug has a broad range of therapeutic areas and active indications, primarily focused on various types of cancers. Its mechanism of action suggests potential in modulating the immune response. The advancement of Favezelimab in clinical development indicates promising results, and its progress in China highlights its potential for the Chinese market.