BioLineRx reveals FDA approval for APHEXDA™ (motixafortide) to stimulate stem cell collection for autologous transplant in Multiple Myeloma patients
BioLineRx Ltd., a corporation at the commercial phase and specialized in biopharmaceuticals, with its main focus on specific forms of cancer and uncommon illnesses, declared today that the APHEXDA™ (motixafortide) along with filgrastim, has received approval from U.S. FDA. This combination is utilized to mobilize hematopoietic stem cells to the peripheral blood, enabling their collection, and further autologous transplantation for patients suffering from multiple myeloma. The administration of APHEXDA is carried out subcutaneously via injection.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Multiple myeloma is the second most-common hematologic malignancy. In treating this particular cancer, Autologous stem cell transplantation stands as a staple in the typical care strategy, providing patients with extended life expectancy. The effectiveness of ASCT is contingent on successfully mobilizing the stem cells throughout the treatment.
"Greater numbers of patients suffering from multiple myeloma are potential candidates for autologous stem cell therapy. Yet, accomplishing set objectives for cell collection can present challenges due to modern obstacles such as treating elderly patients or utilizing updated induction schemes," emphasized John DiPersio, MD, PhD, the leading investigator for the GENESIS trial and Professor of Medicine, Pathology, and Immunology at Washington University's School of Medicine.
The approval given by the FDA for APHEXDA is anchored on outcomes from the two-part, Phase 3 GENESIS trial. This was a randomized, double-blind, placebo-controlled examination that assessed the safety and potency of APHEXDA (motixafortide) used in combination with filgrastim, as opposed to just utilizing the placebo alongside filgrastim, for instigating hematopoietic stem cells for autologous transplantation in patients with multiple myeloma.
The first phase involved a lead-in, single-center, open-label study involving 12 patients who were administered with motixafortide paired with filgrastim with the aim of determining the dosage. The second phase saw 122 patients chosen randomly 2:1 in a double-blind, placebo-controlled, multi-center trial.
Noting the potent efficacy data evident in the GENESIS trial, which incorporated patients that reflect demographic representation of the present multiple myeloma patients, it is our belief that APHEXDA is set to crucially address unfulfilled needs," asserted Philip Serlin, BioLineRx Ltd's Chief Executive Officer. "The company is zealously striving to make this ground-breaking advancement in stem cell mobilization accessible to suitable patients, their medical practitioners, and transplants teams."
"The FDA approval of APHEXDA, being our institution's pioneer-approved therapeutic, signifies a highly thrilling and significant milestone in our journey, hence validating our drug innovation programs," articulated Ella Sorani, PhD, the Chief Development Officer at BioLineRx Ltd. "We are thankful to all our patients and their families for their invaluable contributions towards the research and growth of APHEXDA."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
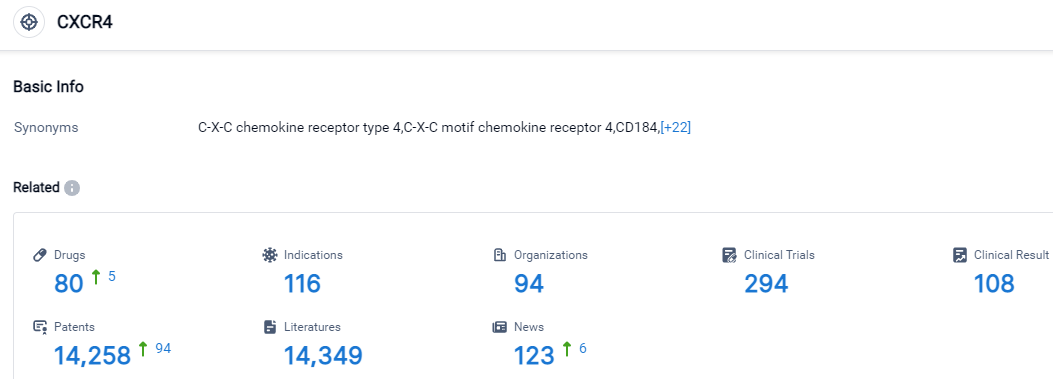
According to the data provided by the Synapse Database, As of September 17, 2023, there are 80 investigational drugs for the CXCR4 target, including 116 applicable indications,94 R&D institutions involved, with related clinical trials reaching 294,and as many as 14258 patents.
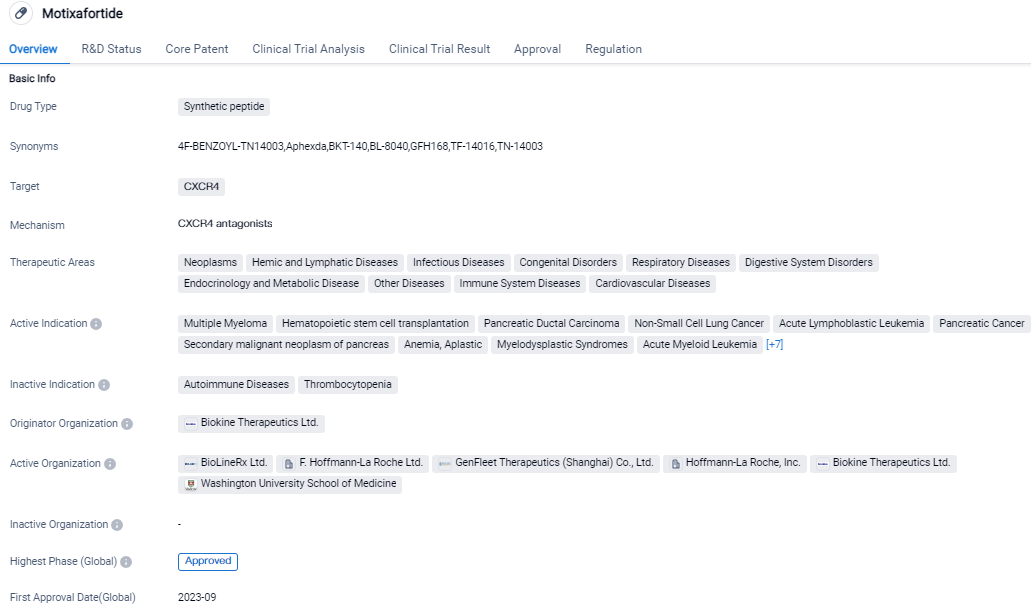
APHEXDA (motixafortide) is a CXCR4 antagonist with long receptor occupancy that, in combination with filgrastim, enables mobilization of hematopoietic stem cells to the peripheral blood for collection and subsequent autologous stem cell transplantation in patients with multiple myeloma. Its approval in the United States and ongoing clinical trials in China indicate its progress in development. As an orphan drug, it holds promise for addressing unmet medical needs in rare diseases or conditions.