An In-depth Analysis of Sotagliflozin's R&D Progress and Mechanism of Action on Drug Target
Sotagliflozin's R&D Progress
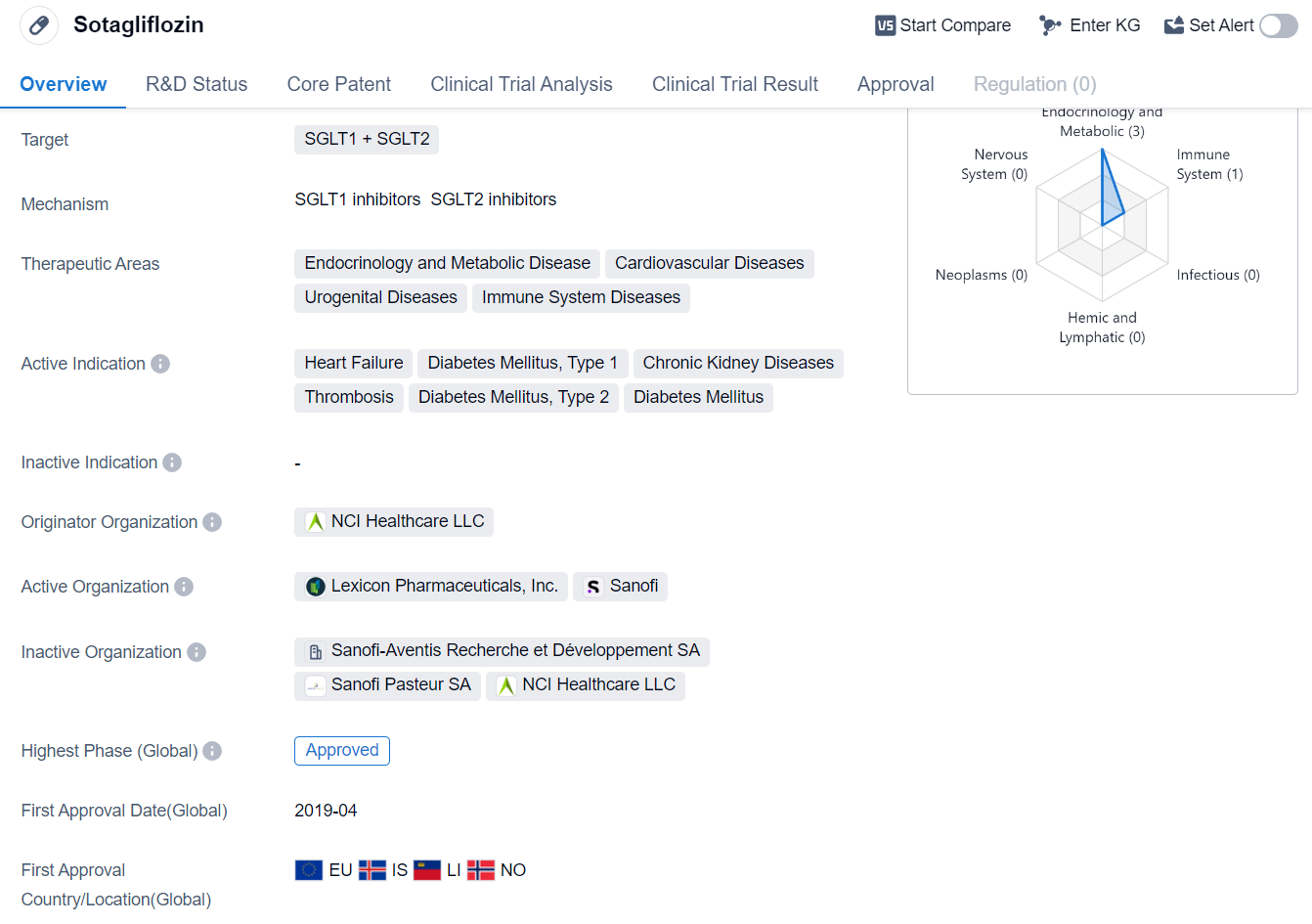
Sotagliflozin is a small molecule drug that targets both SGLT1 and SGLT2. It is primarily used in the treatment of various diseases related to endocrinology and metabolic disorders, cardiovascular diseases, urogenital diseases, and immune system diseases. The drug has been approved for use in several indications, including heart failure, diabetes mellitus type 1 and type 2, chronic kidney diseases, and thrombosis.
The drug was developed by NCI Healthcare LLC, an originator organization in the pharmaceutical industry. It has undergone clinical trials and has reached the highest phase of approval globally. The first approval for Sotagliflozin was granted in April 2019, and it was approved for use in the European Union, Iceland, Liechtenstein, and Norway.
Sotagliflozin's mechanism of action involves targeting both SGLT1 and SGLT2. SGLT1 is responsible for glucose absorption in the gastrointestinal tract, while SGLT2 is involved in glucose reabsorption in the kidneys. By inhibiting both SGLT1 and SGLT2, Sotagliflozin helps to reduce glucose levels in the body, making it an effective treatment for diabetes mellitus.
The drug's approval for heart failure indicates its potential in managing this condition, which is characterized by the heart's inability to pump blood effectively. Additionally, Sotagliflozin's approval for chronic kidney diseases suggests its efficacy in treating kidney-related disorders.
The approval of Sotagliflozin in multiple therapeutic areas highlights its versatility and potential to address various diseases. Its approval in the European Union, Iceland, Liechtenstein, and Norway indicates its acceptance and recognition in these regions.
While Sotagliflozin has reached the highest phase of approval globally, it is still in Phase 1 in China. This suggests that further clinical trials and regulatory processes are required before it can be approved for use in the Chinese market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Sotagliflozin: SGLT1 inhibitor and SGLT2 inhibitor
SGLT1 inhibitors and SGLT2 inhibitors are both types of drugs used in the treatment of diabetes mellitus.
From a biomedical perspective, SGLT1 and SGLT2 are proteins involved in the reabsorption of glucose in the kidneys. SGLT1 is primarily found in the small intestine, where it helps in the absorption of glucose from the diet. SGLT2, on the other hand, is predominantly located in the kidneys, where it reabsorbs glucose from the urine back into the bloodstream.
SGLT1 inhibitors are a class of drugs that specifically target and inhibit the activity of SGLT1 protein in the small intestine. By blocking SGLT1, these inhibitors reduce the absorption of glucose from the diet, leading to lower blood glucose levels.
SGLT2 inhibitors, on the other hand, target and inhibit the activity of SGLT2 protein in the kidneys. By blocking SGLT2, these inhibitors prevent the reabsorption of glucose from the urine, causing increased glucose excretion in the urine and lowering blood glucose levels.
Both SGLT1 and SGLT2 inhibitors are used as adjunctive therapies in the management of type 2 diabetes. They work by different mechanisms to help control blood glucose levels and can be prescribed in combination with other antidiabetic medications. It is important to note that these drugs have distinct mechanisms of action and should not be confused with each other.
Drug Target R&D Trends for Sotagliflozin
Based on the analysis of the provided data, Lexicon Pharmaceuticals, Inc. is the company with the highest stage of development under the target SGLT1 + SGLT2, with drugs in the Approved, NDA/BLA, and Phase 3 stages. Indications such as Heart Failure and Diabetes Mellitus, Type 1 have drugs approved under the target. Small molecule drugs are progressing rapidly, with the highest number of drugs in various stages of development. The countries/locations developing fastest under the target include the United States, European Union, South Korea, South Africa, Germany, United Kingdom, Italy, Denmark, France, Bulgaria, Spain, Belgium, Chile, Czechia, Mexico, Argentina, Brazil, Taiwan Province, Turkey, and Slovakia. Overall, the competitive landscape for the target SGLT1 + SGLT2 is diverse, with multiple companies and countries actively involved in research and development efforts.
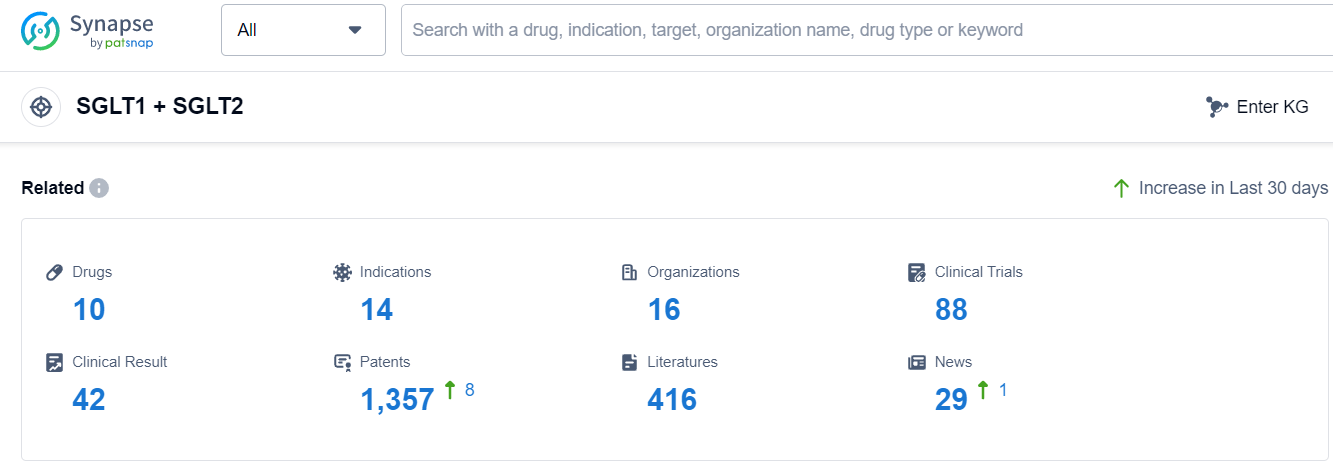
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 10 SGLT1 + SGLT2 drugs worldwide, from 16 organizations, covering 14 indications, and conducting 88 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Sotagliflozin is a small molecule drug that targets SGLT1 and SGLT2. It has been approved for use in various therapeutic areas, including endocrinology and metabolic diseases, cardiovascular diseases, urogenital diseases, and immune system diseases. Developed by NCI Healthcare LLC, the drug has reached the highest phase of approval globally and was first approved in the European Union, Iceland, Liechtenstein, and Norway in April 2019. Its approval for multiple indications, including heart failure and diabetes mellitus, highlights its potential in managing these conditions. However, further development is required for its approval in China.