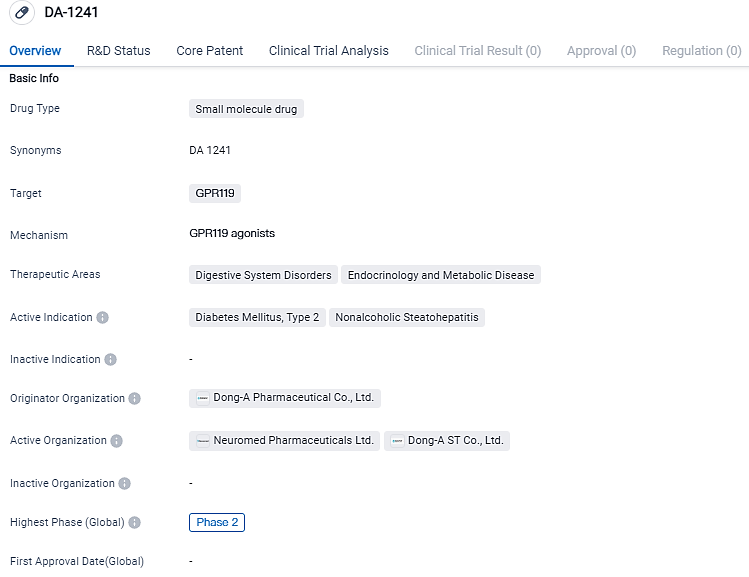
The first patient receives treatment from NeuroBo Pharmaceuticals in their Phase 2a Clinical Trial studying DA-1241
NeuroBo Pharmaceuticals, Inc., a clinical-phase biotech firm aiming to revolutionize cardiometabolic disease treatment, unveils commencement of its Phase 2a clinical trial for the first patient, applying DA-1241, a unique G-Protein-Coupled Receptor 119 (GPR119) agonist, in treating nonalcoholic steatohepatitis. The trial is carried out at The Pinnacle Edinberg/South Texas Research Institute, in Edinburg, Texas, under the oversight of Dr. David Ramirez, the Principal Investigator.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Dosing the first patient on time, reflects our dedication towards the clinical improvement of DA-1241 in NASH patients," claimed Hyung Heon Kim, the CEO and President of NeuroBo. "Based on current clinical and pre-clinical findings, DA-1241 has displayed a decrease in hepatic inflammation, hepatic steatosis, and liver fibrosis. The trial's two-part structure allows the possibility for a preliminary analysis in the first six months of 2024, and we anticipate revealing the full dataset in the last six months of 2024."
Both sections of the Phase 2a exploration of DA-1241 are planned to be 16-week, multiple-location, double-blind, randomized, placebo-controlled, side-by-side studies to determine the safety and effectiveness of DA-1241 in subjects with suspected NASH and confirmed pre-diabetes or T2DM.
Part 1 will aim to determine the effectiveness of DA-1241 against a placebo, expecting to sign up 49 subjects, with a maximum planned enrolment of 55 to account for early dropouts. Subjects will be split into three treatment groups in a 1:2:1 ratio, named: DA-1241 50 mg, DA-1241 100 mg, or placebo.
Part 2 will aim to determine the effectiveness of DA-1241 when combined with sitagliptin, against a placebo, and will commence after the conclusion of a validating preclinical safety study of DA-1241 and sitagliptin. Expectation of enrollment is 37 subjects, with a maximum planned enrollment of 43 to account for early dropouts, and subjects will be segregated into 2 treatment groups: DA-1241 100 mg/sitagliptin 100 mg or placebo, in a 2:1 ratio.
The primary endpoint for both parts 1 and 2, is the change in alanine transaminase levels from the starting point at the 16th week. Other secondary endpoints to assess the efficacy include the proportion of subjects with normalization of ALT, absolute change in total cholesterol, high and low-density lipoprotein cholesterol, triglyceride, and free fatty acids from the starting point and other amongst other things. Safety will be determined by monitoring AEs, SAEs and AEs resulting in discontinuation and abnormalities in the lab results.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
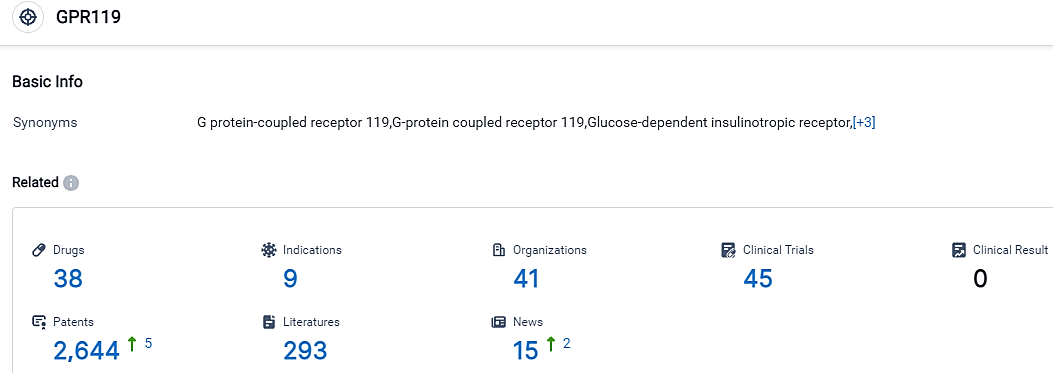
According to the data provided by the Synapse Database, As of September 19, 2023, there are 38 investigational drugs for the GPR119 target, including 9 indications,41 R&D institutions involved, with related clinical trials reaching 45,and as many as 2644 patents.
DA-1241 is a novel G-Protein-Coupled Receptor 119 agonist with development optionality as a standalone and/or combination therapy for both NASH and T2DM. In preclinical studies, DA-1241 demonstrated that GPR-119 agonism promotes the release of the key gut peptides GLP-1, GIP, and PYY, which have a beneficial effect on liver inflammation, lipid metabolism, weight loss, and glucose metabolism. Currently in Phase 2, DA-1241 is undergoing clinical trials to evaluate its safety and efficacy.