The US has sanctioned Ojjaara (momelotinib), the the first and only therapeutic agent for anemia
GSK plc has confirmed that Ojjaara(momelotinib), a daily oral JAK1/JAK2 and activin A receptor type 1 inhibitor, has received approval from the US FDA. This approval authorizes its use for the treatment of intermediate or high-risk myelofibrosis, with cases inclusive of primary myelofibrosis or secondary myelofibrosis, in adults suffering from anemia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Until now, it is the only approved drug for both recently identified and formerly treated myelofibrosis patients with anemia that tackles the primary effects of the illness; specifically anemia, usual symptoms, and splenomegaly.
Nina Mojas, GSK’s Senior Vice President of Oncology Global Product Strategy, commented: “Most myelofibrosis sufferers gradually encounter anemia, leading them to discontinue therapy and call for transfusions. In light of this pressing need, we are delighted to incorporate Ojjaara into our oncology line-up, meeting an important healthcare requirement in the society. We look forward to helping improve outcomes in this difficult-to-treat blood cancer.”
Myelofibrosis, a type of blood malignancy, impacts almost 25,000 patients in America. This condition can result in critically low blood counts, such as anemia and thrombocytopenia; usual symptoms like fatigue, nocturnal sweats, and bone pain; and splenomegaly. Approximately 40% of these patients suffer from moderate to severe anemia upon being diagnosed, and almost all patients are conjectured to encounter anemia through the progression of the disease. Those who are dependent on transfusions have a grim prognosis and a reduced lifespan.
The FDA approval of momelotinib is supported by data from the crucial MOMENTUM study, along with a subpopulation of adult sufferers with anemia from the SIMPLIFY-1 phase III trial. MOMENTUM was constructed to estimate the safety and efficiency of momelotinib compared to danazol for the management and reduction of myelofibrosis' primary effects in anemic, symptomatic, JAK inhibitor-experienced population.
Kapila Viges, the Chief Executive Officer of MPNResearch Foundation, expressed: "We are excited to witness momelotinib making its way to the clinic. This furnishes patients and their doctors another choice to control myelofibrosis. Any novel therapy taking strides towards understanding this intricate and chronic blood cancer marks significant headway for the field."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
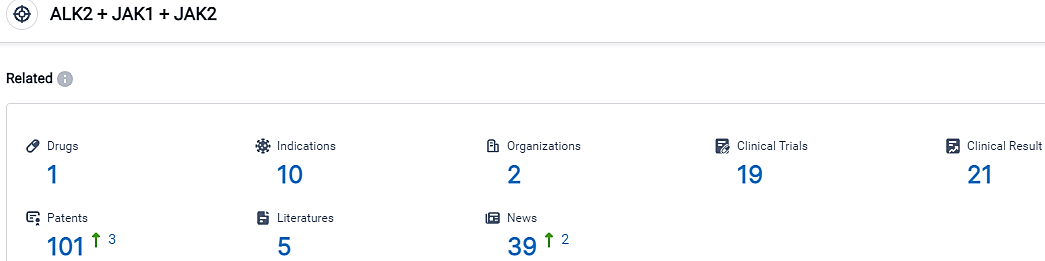
According to the data provided by the Synapse Database, As of September 19, 2023, there are 1 investigational drugs for the ALK2+JAK1+JAK2 target, including 10 indications,2 R&D institutions involved, with related clinical trials reaching 19,and as many as 101 patents.
Ojjaarahas a differentiated mechanism of action, with inhibitory ability along three key signaling pathways: Janus kinase 1, JAK2, and activin A receptor, type I. Inhibition of JAK1 and JAK2 may improve constitutional symptoms and splenomegaly. Additionally, inhibition of ACVR1 leads to a decrease in circulating hepcidin, which is elevated in myelofibrosis and contributes to anemia.