Selumetinib: Detailed Review of its Transformative R&D Success
Selumetinib's R&D Progress
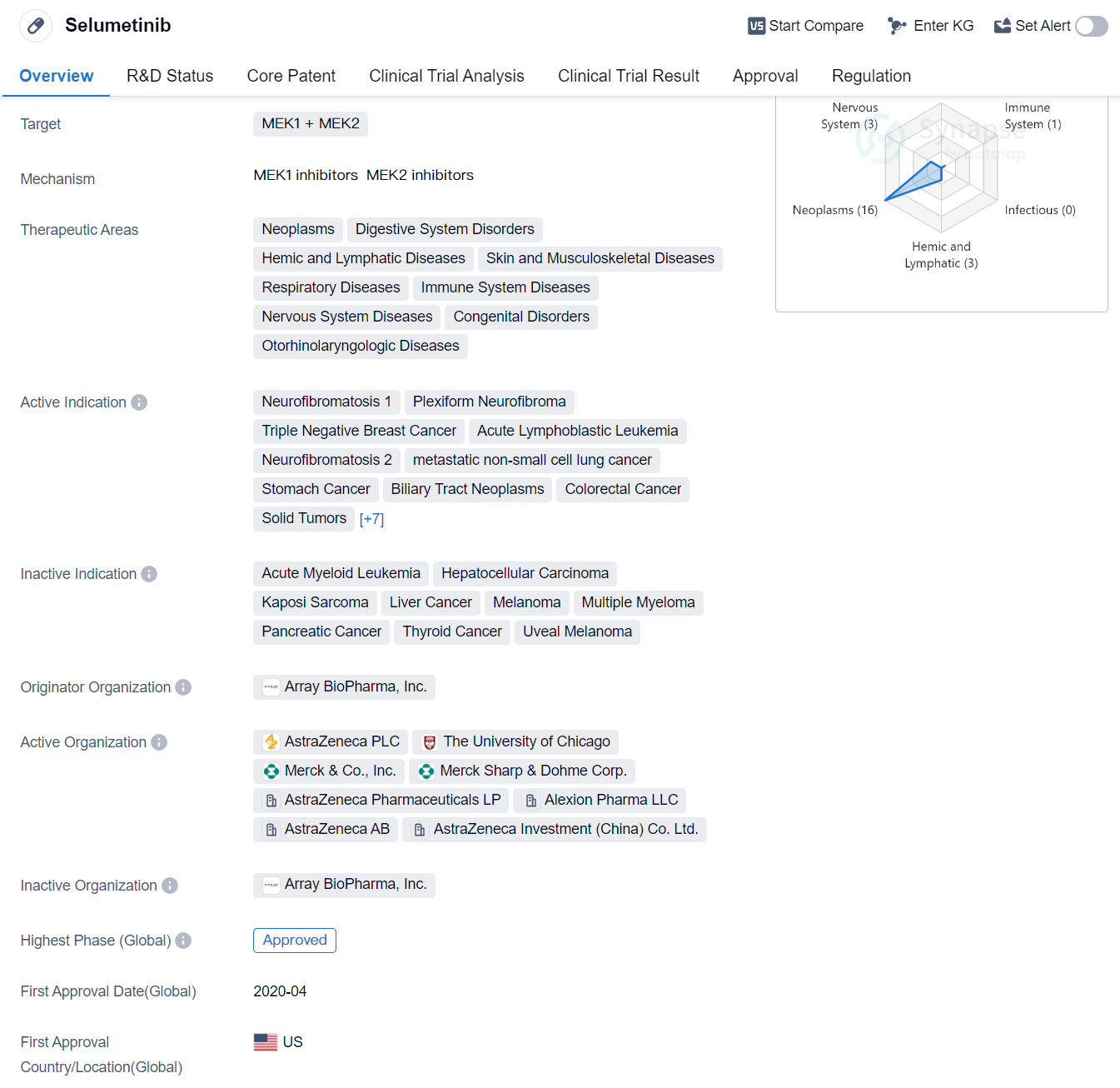
Selumetinib is a small molecule drug that targets MEK1 and MEK2. It has been approved for use in various therapeutic areas including neoplasms, digestive system disorders, hemic and lymphatic diseases, skin and musculoskeletal diseases, respiratory diseases, immune system diseases, nervous system diseases, congenital disorders, and otorhinolaryngologic diseases.
The drug has shown efficacy in treating several active indications such as neurofibromatosis 1, plexiform neurofibroma, triple negative breast cancer, acute lymphoblastic leukemia, neurofibromatosis 2, metastatic non-small cell lung cancer, stomach cancer, biliary tract neoplasms, colorectal cancer, solid tumors, chronic myelogenous leukemia, primary myelofibrosis, breast cancer, esophageal carcinoma, lung cancer, squamous cell carcinoma of the head and neck, and non-small cell lung cancer.
Selumetinib was developed by Array BioPharma, Inc., and it has been approved in the global market. The drug obtained its first approval in the United States in April 2020. It has undergone regulatory processes such as priority review, orphan drug designation, and breakthrough therapy designation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Selumetinib: MEK1 inhibitor and MEK2 inhibitor
MEK1 inhibitors and MEK2 inhibitors are types of drugs that target and inhibit the activity of MEK1 and MEK2 enzymes. MEK1 and MEK2 are protein kinases that are part of the mitogen-activated protein kinase (MAPK) signaling pathway, which plays a crucial role in cell growth, proliferation, and survival.
From a biomedical perspective, MEK1 and MEK2 inhibitors are used in the treatment of certain types of cancer, particularly those that have mutations or dysregulation in the MAPK pathway. By inhibiting the activity of MEK1 and MEK2, these inhibitors disrupt the signaling cascade and prevent the activation of downstream targets involved in tumor growth and progression.
MEK1 and MEK2 inhibitors are often used in combination with other targeted therapies or chemotherapy drugs to enhance their effectiveness. They have shown promising results in the treatment of various cancers, including melanoma, non-small cell lung cancer, and colorectal cancer.
It is important to note that MEK1 and MEK2 inhibitors can have side effects, including skin rash, diarrhea, fatigue, and elevated liver enzymes. Close monitoring and management of these side effects are necessary during treatment.
Overall, MEK1 inhibitors and MEK2 inhibitors are valuable tools in the field of biomedicine for targeted cancer therapy, specifically for cancers associated with dysregulated MAPK signaling pathway.
Drug Target R&D Trends for Selumetinib
The analysis of the current competitive landscape and future development of target MEK1 + MEK2 reveals that Pfizer Inc., AstraZeneca PLC, Roche Holding AG, Novartis AG, and Merck & Co., Inc. are the companies growing the fastest under this target. The approved drugs under this target cover a wide range of indications, indicating the potential of MEK1 + MEK2 inhibitors in treating various types of cancer. Small molecule drugs dominate the market, but the presence of monoclonal antibodies, PROTACs, and molecular glue suggests the exploration of innovative drug types. The development of drugs targeting MEK1 + MEK2 is progressing rapidly in countries/locations such as the United States, European Union, Japan, and China. Overall, the target MEK1 + MEK2 presents a competitive landscape with potential for future development and innovation in the pharmaceutical industry.
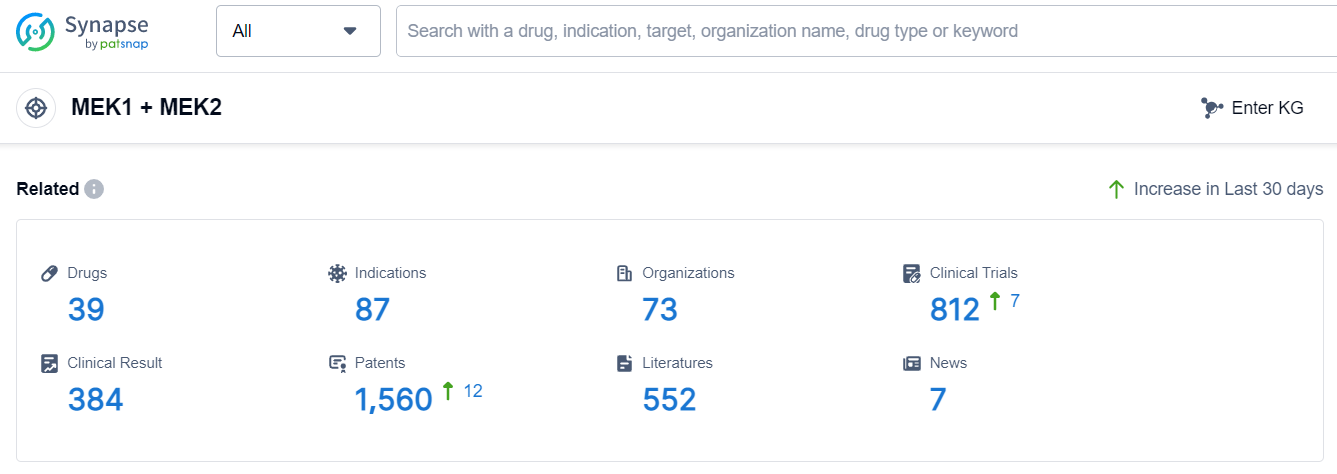
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 39 MEK1 + MEK2 drugs worldwide, from 73 organizations, covering 87 indications, and conducting 812 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Selumetinib is a small molecule drug that targets MEK1 and MEK2. It has been approved for various therapeutic areas and has shown efficacy in treating multiple active indications. Developed by Array BioPharma, Inc., the drug has received global approvals. Its first approval was granted in the United States in April 2020, and it has undergone regulatory processes such as priority review, orphan drug designation, and breakthrough therapy designation.