An Overview of Bausch's 230 Drug Pipelines ——Top 50 Pharmaceutical Companies R&D Progress
Bausch Health Cos., Inc., formerly known as Valeant Pharmaceuticals International, is a pharmaceutical company founded in 1959 and headquartered in Quebec, Canada. The company operates in the field of biomedicine and has a diverse portfolio of drugs targeting various therapeutic areas.
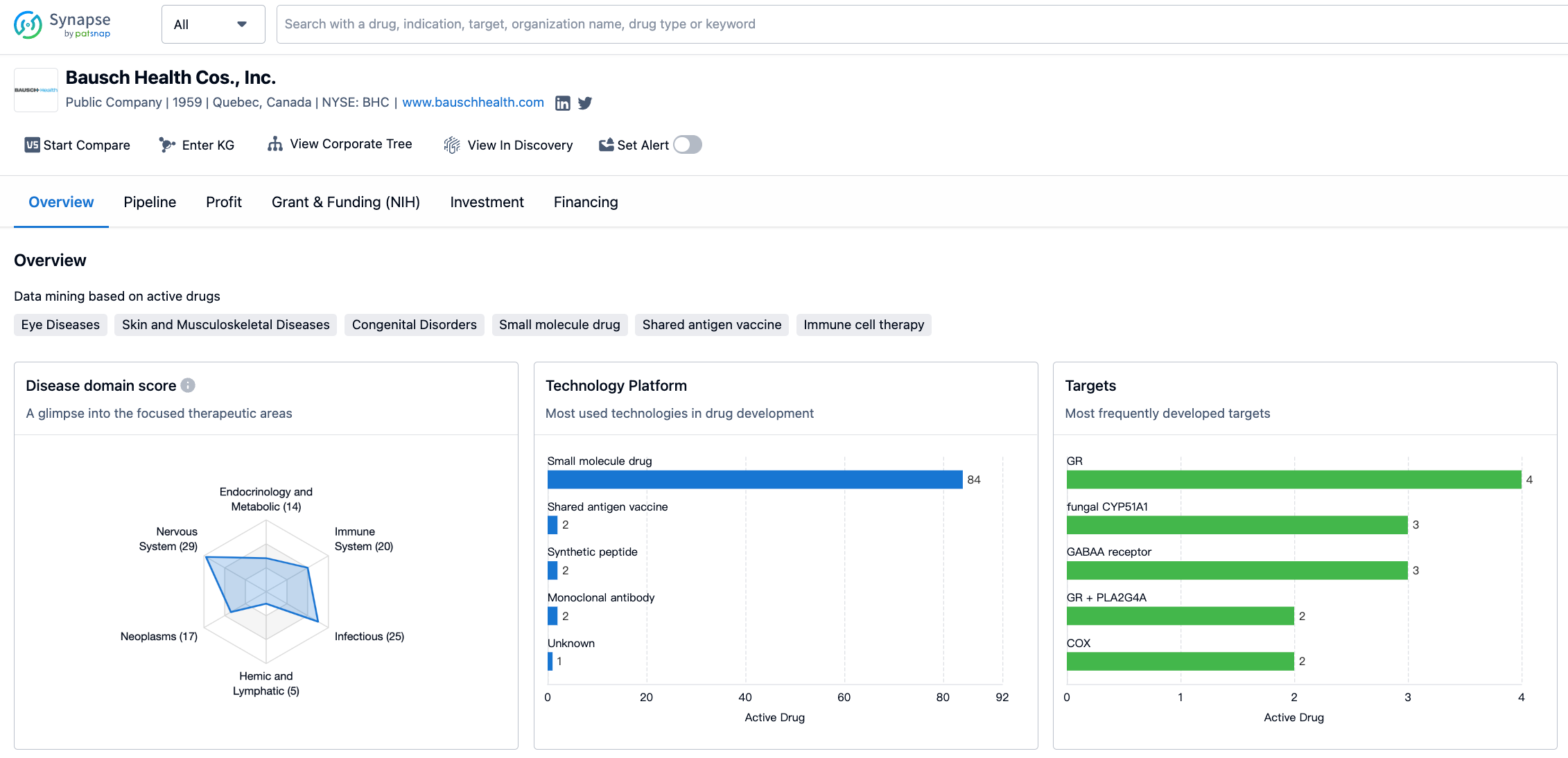
According to the distribution of therapeutic areas, Bausch Health Cos., Inc. has a significant focus on skin and musculoskeletal diseases, with 36 drugs in its portfolio. Other diseases, nervous system diseases, infectious diseases, and eye diseases are also areas of emphasis for the company, with drug counts of 31, 29, 25, and 23 respectively. Immune system diseases, neoplasms, and digestive system disorders are also areas where Bausch Health Cos., Inc. has a notable presence.
In terms of the most frequently developed targets, Bausch Health Cos., Inc. has focused on several key targets. The glucocorticoid receptor (GR) is the most frequently targeted, with 4 drugs developed. Other targets include fungal CYP51A1, GABAA receptor, GR + PLA2G4A, COX, mAChRs, 50S subunit, GC-C, and PTGFR, among others. These targets represent a range of therapeutic areas and indicate the company's commitment to addressing various diseases and conditions.
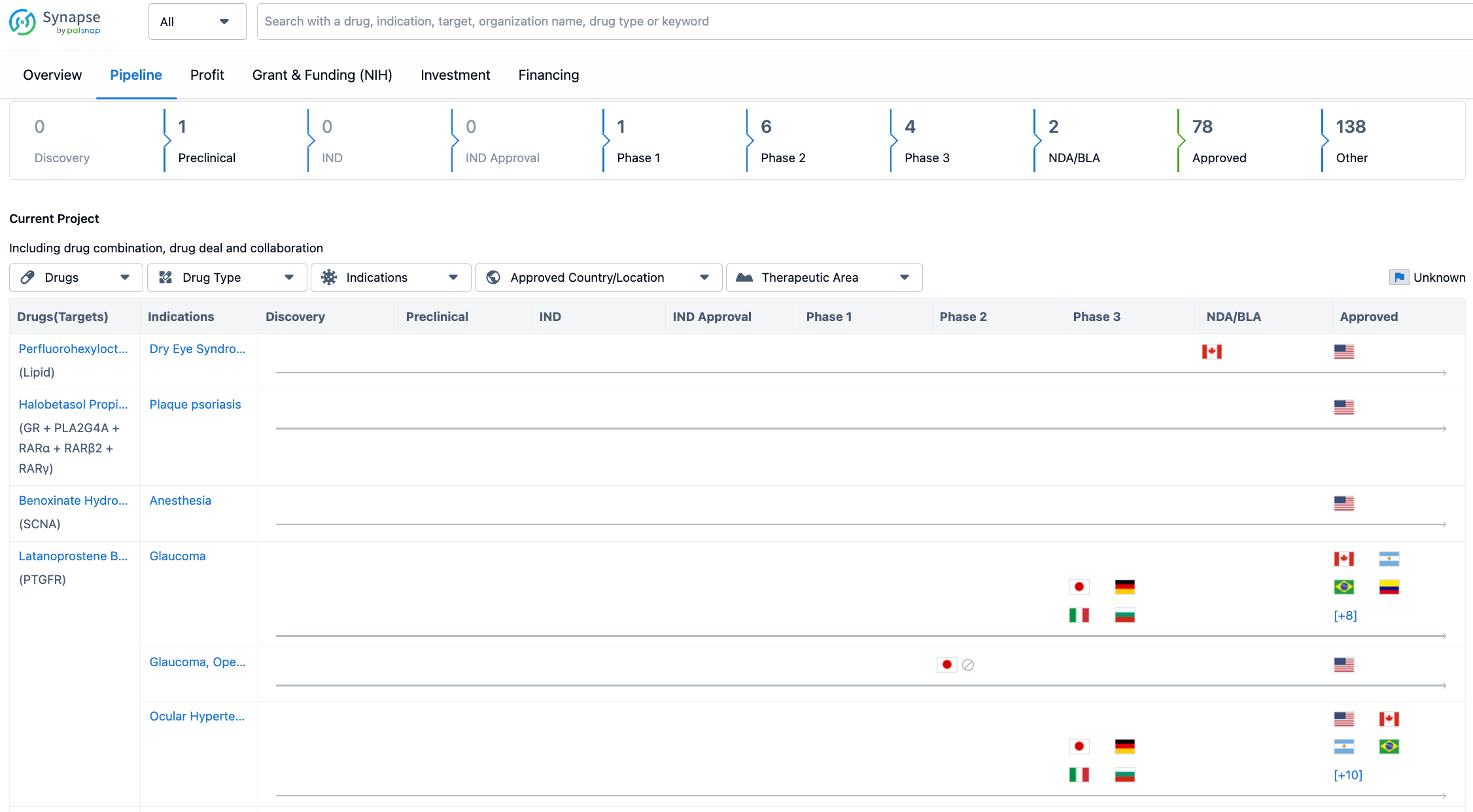
Looking at the pipeline for Bausch Health Cos., Inc. till 2023-07-26, it is evident that the company has a significant number of drugs in advanced stages of development. The pipeline includes drugs in preclinical, Phase 1, Phase 2, Phase 3, NDA/BLA, and approved stages. Notably, there are 78 drugs that have already received approval, indicating the company's success in bringing new treatments to market.
In terms of the distribution of drugs across different phases, the majority of drugs are in the approved stage, followed by the other category, which likely includes drugs that are in earlier stages of development or have not yet progressed to clinical trials. Among the clinical stages, Phase 2 has the highest number of drugs, with 6 in development, followed by Phase 3 with 4 drugs. The presence of drugs in Phase 1 indicates ongoing research and development efforts by Bausch Health Cos., Inc.
The information provided gives an overview of Bausch Health Cos., Inc.'s activities in the pharmaceutical industry. The company has a diverse portfolio of drugs targeting various therapeutic areas, with a particular focus on skin and musculoskeletal diseases, other diseases, nervous system diseases, and infectious diseases. The most frequently developed targets include the glucocorticoid receptor, fungal CYP51A1, and GABAA receptor, among others.
The pipeline for Bausch Health Cos., Inc. indicates a significant number of drugs in advanced stages of development, with a substantial number already approved. This suggests that the company has a strong track record in bringing new treatments to market. The presence of drugs in earlier stages of development indicates ongoing research and development efforts by the company.
Overall, Bausch Health Cos., Inc. is a pharmaceutical company with a diverse portfolio of drugs targeting various therapeutic areas. The company's focus on research and development is evident from its pipeline, which includes drugs in different stages of development. With its long history in the industry and a strong presence in the market, Bausch Health Cos., Inc. continues to contribute to the advancement of biomedicine and the treatment of various diseases and conditions.