An Overview of Biogen's 137 Drug Pipelines ——Top 50 Pharmaceutical Companies R&D Progress
Biogen, Inc. is a pharmaceutical company that was founded in 1978 and is headquartered in Massachusetts, United States. The company operates in the field of biomedicine and focuses on the development of drugs for various therapeutic areas.
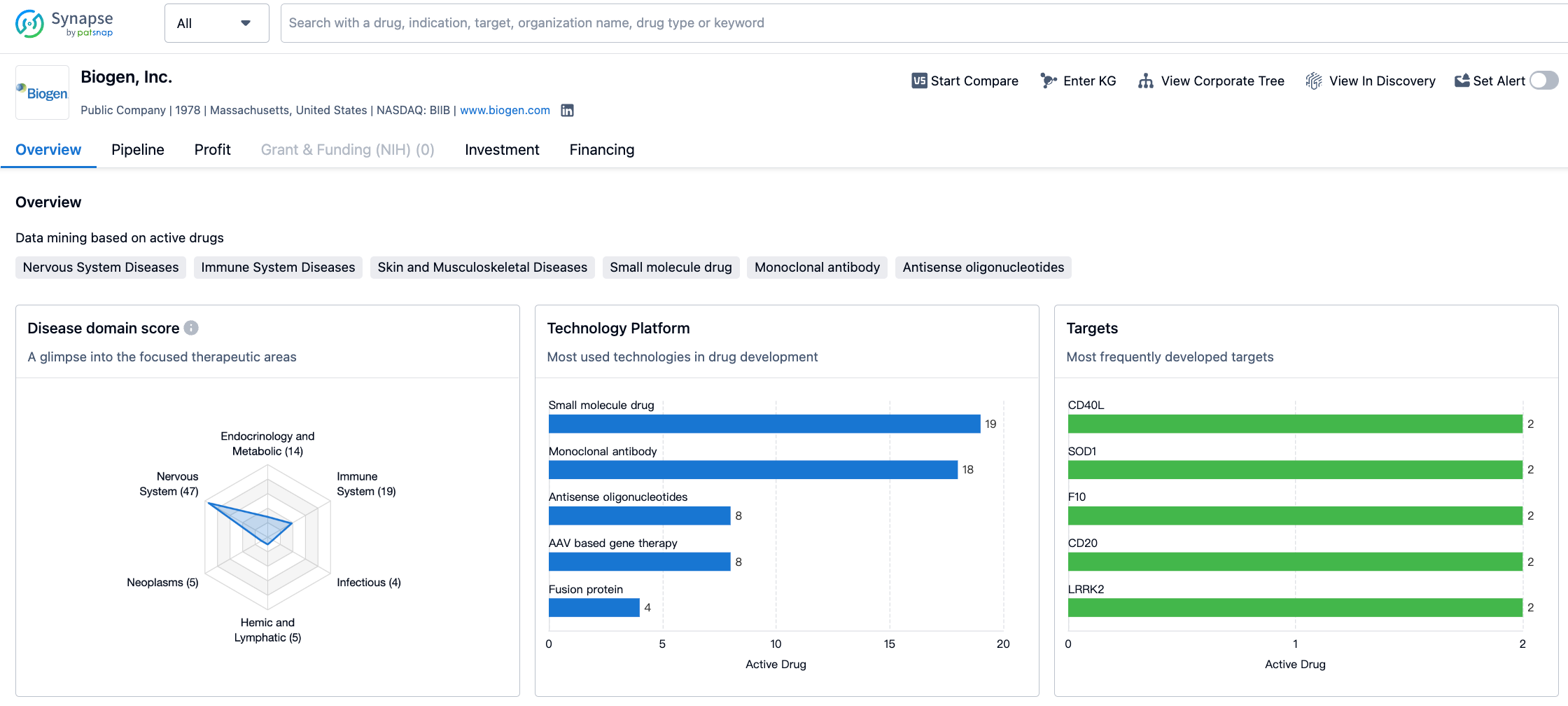
Biogen has a diverse portfolio of drugs that target different therapeutic areas. The company has the highest number of drugs in the Nervous System Diseases category, with a count of 47. This indicates that Biogen has a strong focus on developing treatments for neurological disorders. The second highest number of drugs is in the Immune System Diseases category, with 19 drugs.
Other therapeutic areas that Biogen is involved in include Endocrinology and Metabolic Disease (14 drugs), Skin and Musculoskeletal Diseases (12 drugs), Congenital Disorders (8 drugs), and Eye Diseases (7 drugs). The company also has a presence in areas such as Hemic and Lymphatic Diseases, Neoplasms, Digestive System Disorders, Infectious Diseases, Urogenital Diseases, Cardiovascular Diseases, Mouth and Tooth Diseases, and Respiratory Diseases, although the drug count is relatively lower in these areas.
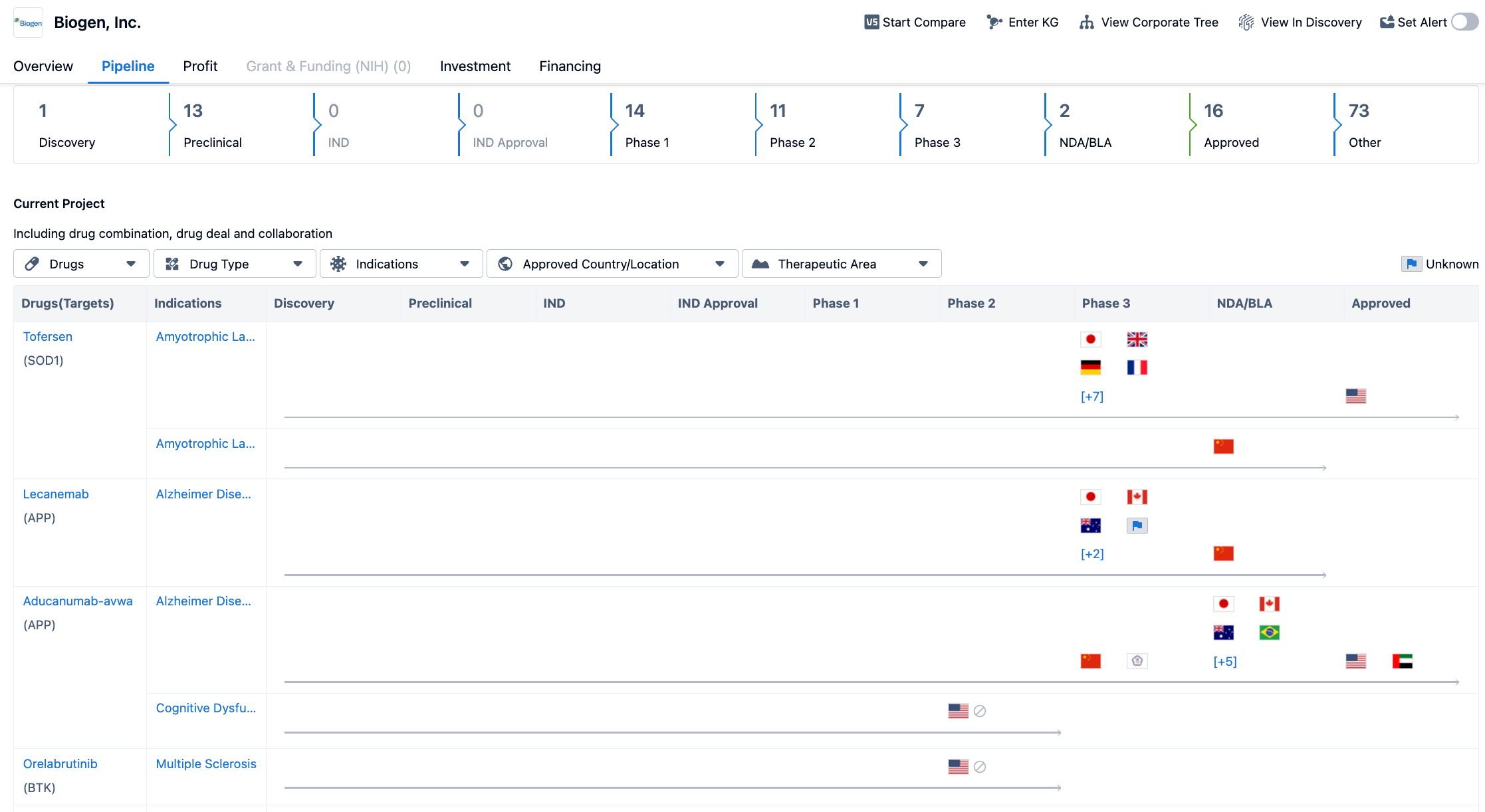
The pipeline is categorized into different phases, starting from Discovery to Approved. The pipeline includes 1 drug in the Discovery phase, 13 drugs in the Preclinical phase, 14 drugs in Phase 1, 11 drugs in Phase 2, 7 drugs in Phase 3, 2 drugs in NDA/BLA (New Drug Application/Biologics License Application), 16 drugs that have been Approved, and 73 drugs categorized as Other.
Biogen has a strong focus on the development of drugs for neurological and immune-related disorders. The company has a significant number of drugs in the Nervous System Diseases and Immune System Diseases categories, indicating its commitment to addressing these therapeutic areas. Additionally, Biogen is actively developing drugs that target specific proteins and receptors.
In conclusion, Biogen, Inc. is a pharmaceutical company that has been operating in the biomedicine field since 1978. The company has a strong focus on developing drugs for neurological and immune-related disorders, as evidenced by the high drug count in the Nervous System Diseases and Immune System Diseases categories. Biogen is actively developing drugs that target specific proteins and receptors, and its pipeline indicates ongoing research and development efforts across various stages of drug development. Overall, Biogen's commitment to addressing unmet medical needs in these therapeutic areas positions it as a key player in the pharmaceutical industry.