Betibeglogene autotemcel - The most expensive medicine in the world
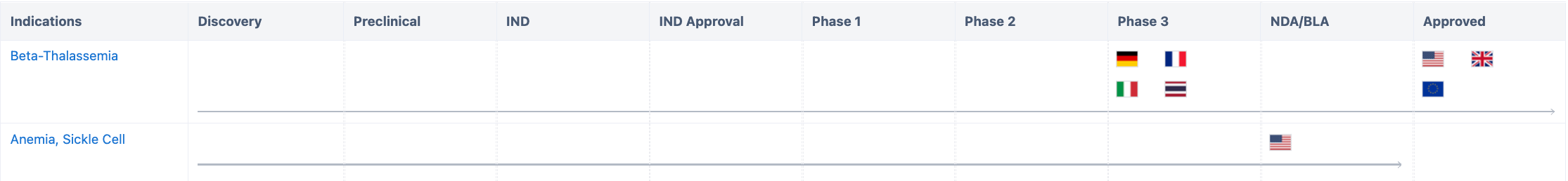
Betibeglogene autotemcel, also known as Lovotibeglogene autotemcel and marketed as ZYNTEGLO, is an intravenous injection developed by bluebird bio, Inc. This stem cell therapy targets β-globin and was first approved in the European Union on May 29, 2019, for the treatment of transfusion-dependent β-thalassemia. On June 22, 2023, the FDA included ZYNTEGLO in its priority review program for the treatment of patients aged 12 and above with a history of pain complications associated with sickle cell disease (SCD). ZYNTEGLO is a one-time gene therapy with the potential to cure SCD.
According to “Synapse”, this drug is focused on the development for Beta-Thalassemia and Sickle Cell Anemia. For more detailed information on R&D status, core patents, analysis, etc., please click on the image link below.
Third ex-vivo gene therapy
If approved, ZYNTEGLO will be bluebird’s third ex-vivo gene therapy approved in the US for a rare genetic disease, and its second FDA approval for an inherited haemoglobin disorder.
Affecting approximately 100,000 people in the US, SCD is a life-long, incurable genetic disease that causes red blood cells to take a distinct crescent shape, which can block blood vessels and affect the way oxygen is carried around the body. The disease can cause serious health problems including anaemia, fatigue, episodes of pain and chronic end-organ damage.
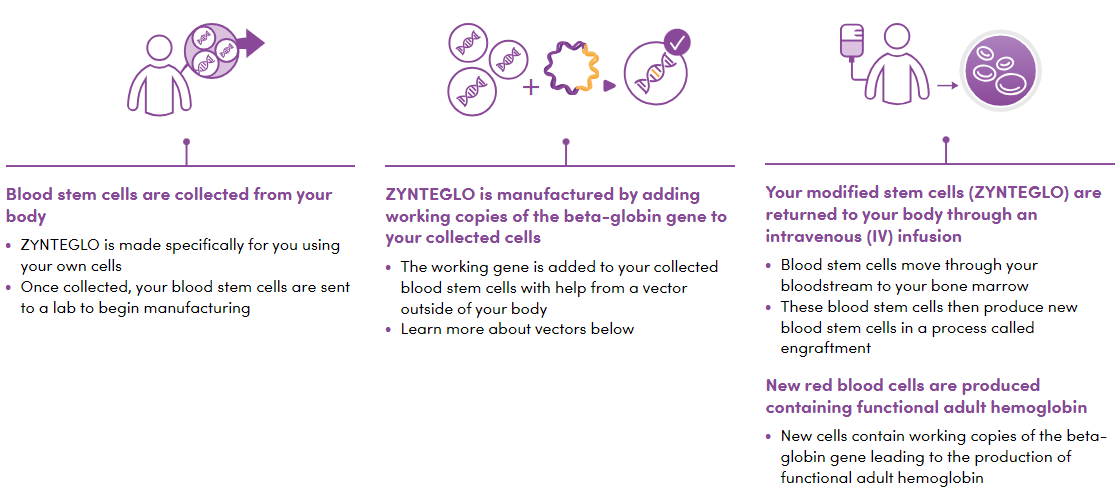
ZYNTEGLO is an investigational one-time treatment designed to add functional copies of a modified form of the beta-globin gene into a patient’s own haematopoietic stem cells.
Efficacy
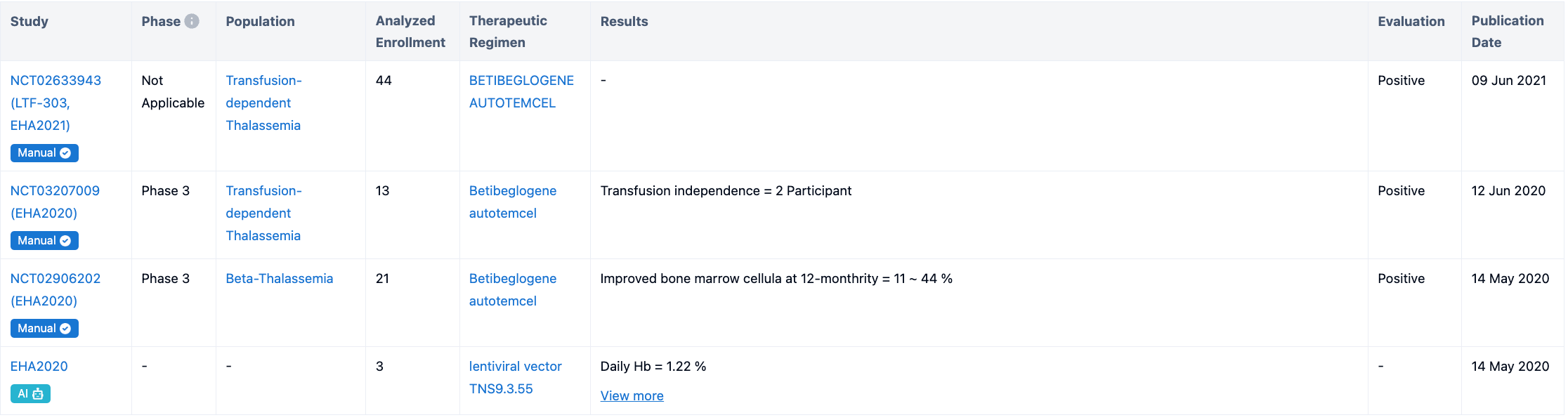
The Definition of TI: Transfusion independence (TI) meant that patients achieved a weighted average hemoglobin of ≥9 g/dL without any transfusions for a continuous period of ≥12 months at any time during the study after infusion of ZYNTEGLO. In two phase 3 studies, ZYNTEGLO was studied in 41 patients, and about 9 out of 10 (89%) patients treated with ZYNTEGLO stopped transfusions. The majority of patients achieved TI (89%; 32/36 patients) and had a normal to near-normal median total hemoglobin of 11.5 g/dL.
Safety
The most common side effects of ZYNTEGLO on the day of treatment are: Increased heart rate and abdominal pain. The most common side effects of ZYNTEGLO following treatment for up to 6 months are a. Low level of platelets, which may reduce the ability of blood to clot and may cause bleeding; b. Low level of white blood cells, which may make you more susceptible to infection; c. Pain in arms or legs.