Analysis on the Clinical Research Progress of CYP2C9 Inhibitors
CYP2C9 is a member of the Cytochrome P450 superfamily of enzymes that primarily metabolize drugs in the human body. It predominantly occurs in the liver and intestine. Approximately 16% of clinically used drugs are metabolized through it, including anti-diabetic drugs such as tolbutamide, glibenclamide, and glimepiride; anti-epileptic drugs like phenytoin; anticoagulants such as warfarin, disulfiram, nitrophenylbenzene, and propylbenzene; antihypertensives like losartan and irbesartan; diuretics like torasemide; antidepressants such as amitriptyline and fluoxetine; non-steroidal anti-inflammatory drugs like ibuprofen, celecoxib, meloxicam, piromelate, and fluorobiphenyl; and antineoplastic drugs like cyclophosphamide and oxazaphosphorines.
To date, a variety of mutant alleles from CYP2C9*2 to CYP2C9*35 have been discovered in CYP2C9, with wild-type CYP2C9*1 and mutant-types CYP2C9*2 and CYP2C9*3 being the most common. The frequency of CYP2C9*2 and CYP2C9*3 genetic mutations varies significantly among different populations, with the mutation incidence among Caucasians being higher than that of Asians and Africans.
Based on the CYP2C9 genotype, individuals can be classified into normal metabolizers, intermediate metabolizers, and slow metabolizers.
CYP2C9 Competitive Landscape
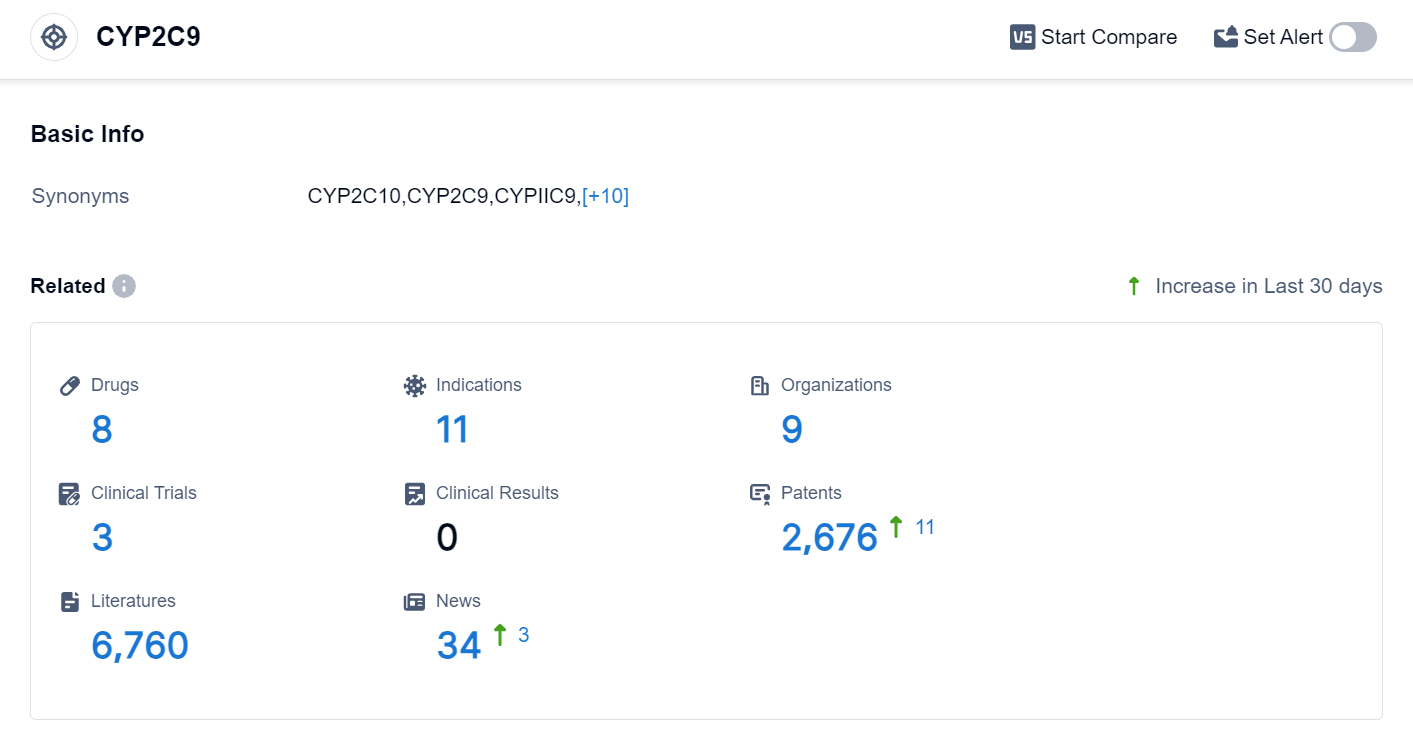
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 24 Sep 2023, there are a total of 8 CYP2C9 drugs worldwide, from 9 organizations, covering 11 indications, and conducting 3 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the current competitive landscape and future development of target CYP2C9 reveals several key findings. Alvogen, Inc., Bayer AG, and Shaanxi Xiangju Pharmaceutical Co. Ltd. are the companies that are growing fastest under the current target, having reached the approved stage of development. These companies have demonstrated significant progress in their R&D efforts.
Drugs targeting CYP2C9 have been approved for indications such as dermatomycoses, mycoses, candidiasis, infectious diseases, hyperuricemia, vaginitis, and skin diseases, infectious. This indicates a recognized need for treatments in these therapeutic areas and the potential efficacy of drugs targeting CYP2C9.
Small molecule drugs are progressing most rapidly under the current target, suggesting a focus on traditional pharmaceutical compounds. This may be due to the advantages of small molecule drugs in terms of formulation, delivery, and cost-effectiveness.
China, the United States, and Japan are the countries/locations developing fastest under the current target. China, in particular, has shown significant progress, indicating the growing capabilities of Chinese pharmaceutical companies in this area.
Overall, the analysis suggests a competitive landscape with companies making progress in R&D efforts, drugs being approved for relevant indications, a focus on small molecule drugs, and the involvement of multiple countries in the development of drugs targeting CYP2C9. The future development of target CYP2C9 holds promise for the advancement of treatments in various therapeutic areas.
The globally approved CYP2C9 inhibitor on the market: Isoconazole Nitrate
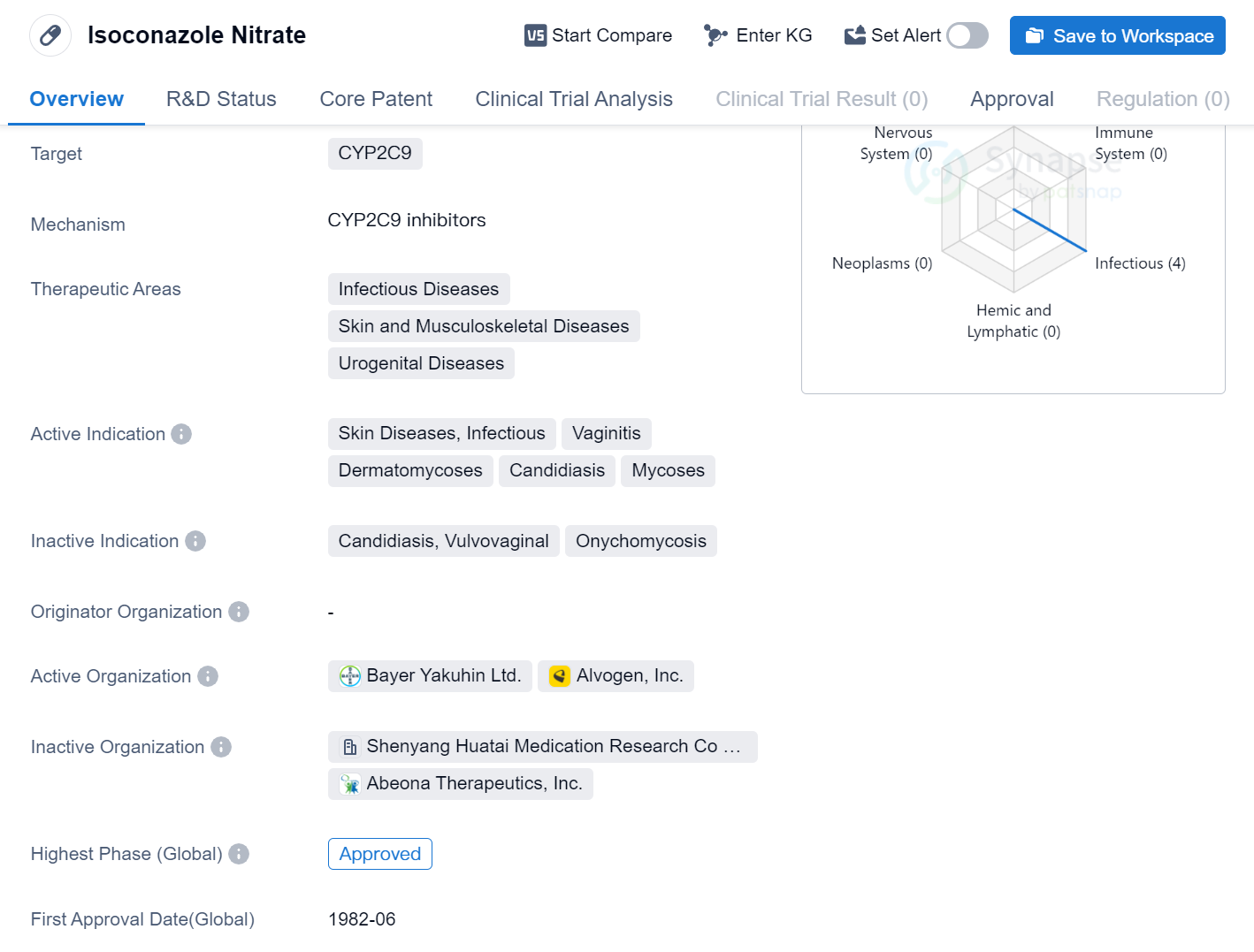
Isoconazole Nitrate is a small molecule drug that targets the enzyme CYP2C9. It is primarily used in the treatment of infectious diseases, skin and musculoskeletal diseases, and urogenital diseases. The drug is indicated for various conditions including skin diseases, infectious vaginitis, dermatomycoses, candidiasis, and mycoses.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Isoconazole Nitrate has reached the highest phase of development, which is approved globally. The drug received its first approval in June 1982 in Japan. This indicates that Isoconazole Nitrate has been in use for several decades and has gained regulatory approval in multiple countries.
As a small molecule drug, Isoconazole Nitrate is likely to have a well-defined chemical structure and can be easily synthesized. It is designed to interact with the CYP2C9 enzyme, which plays a crucial role in drug metabolism. By targeting this enzyme, Isoconazole Nitrate may modulate its activity and potentially provide therapeutic benefits in the treatment of various diseases.
The therapeutic areas of infectious diseases, skin and musculoskeletal diseases, and urogenital diseases suggest that Isoconazole Nitrate may have broad-spectrum activity against different types of pathogens. It may be effective against bacterial, fungal, or viral infections that affect the skin, musculoskeletal system, or urogenital tract.
The active indications of Isoconazole Nitrate, such as skin diseases, infectious vaginitis, dermatomycoses, candidiasis, and mycoses, further highlight its potential in treating specific conditions within the therapeutic areas mentioned earlier. These indications suggest that Isoconazole Nitrate may be used to treat various skin infections, vaginal infections, and fungal diseases.
Overall, Isoconazole Nitrate is an approved small molecule drug that targets the CYP2C9 enzyme. It has been used since 1982 and is indicated for the treatment of infectious diseases, skin and musculoskeletal diseases, and urogenital diseases. Its active indications include skin diseases, infectious vaginitis, dermatomycoses, candidiasis, and mycoses.