SpyBiotech reports submission of Clinical Trial Application to MHRA for SPYVLP01
SpyBiotech, a biotech firm equipped with a unique vaccination platform technology that tackles infectious ailments, cancer, and chronic conditions, has declared that they've submitted a clinical trial request to the U.K. MHRA for SPYVLP01 assessment. This is a vaccine that aims at human cytomegalovirus utilizing its Hepatitis B virus-like-particle platform technology. Currently, there is no sanctioned HCMV vaccine on the market
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Sumi Biswas, Ph.D., President and Chief Scientific Officer at SpyBiotech, stated, "Our clinical trial application submission for SPYVLP01 signifies critical advancement in combating the HCMV virus, a prime infectious catalyst for congenital anomalies. We are confident in the possible benefits of our SpyVLP vaccine platform. Even though our main program targets HCMV, the operative scope of our vaccine technology allows for the creation of immunizations for a broad spectrum of pathogens and treatments."
Mark Leuchtenberger, CEO of SpyBiotech, expressed that "Our application to MHRA for trial progression represents a significant leap forward in evolving our affordable and highly extensible immunization technology.” He further emphasized the urgency for medical breakthroughs in HCMV as there is no current approved vaccine counteracting this betaherpesvirus, which could cause persistent infection in people.
The company's phase 1 clinical trial seeks primarily to scrutinize multiple formulations of their HCMV vaccine. The plan is to enroll up to 120 participants, with the first expected to receive a dose later this year and clinical data to emerge in the latter part of 2024.
The SpyVLP vaccine platform by SpyBiotech is innovative and uses an exclusive protein 'super-adhesive' technology. This technology enables antigens to be attached to vaccine distribution systems in a manner that reduces the risk of delivery and amplifies immune response and effectiveness.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
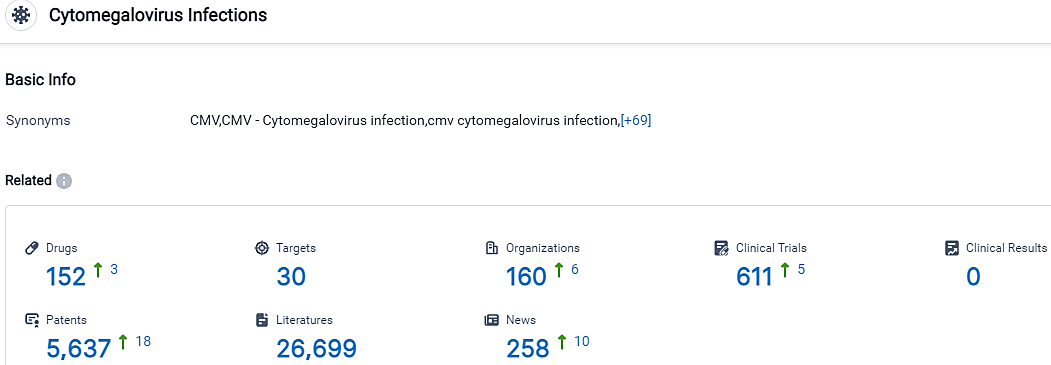
According to the data provided by the Synapse Database, As of September 26, 2023, there are 152 investigational drugs for the Cytomegalovirus Infections, including 30 targets,160 R&D institutions involved, with related clinical trials reaching 611,and as many as 5637 patents.
The SPYVLP-01 vaccine has been formulated by Spybiotech Ltd. to counteract cytomegalovirus infections. At present, it is undergoing the Investigational New Drug application stage, a crucial step in its developmental journey. The creation of a vaccine specifically for CMV infections could fulfil a crucial healthcare requirement and better the prognosis for those susceptible to complications arising from this particular viral infection.