Analysis on the Clinical Research Progress of PD-1 Agonist
PD-1, or programmed cell death protein 1, is a crucial immune checkpoint receptor found on the surface of certain immune cells in the human body. Its primary role is to regulate the immune response and prevent excessive activation of immune cells, thereby maintaining immune homeostasis. PD-1 acts as a brake on the immune system by binding to its ligands, PD-L1 and PD-L2, which are expressed on various cells, including cancer cells. This interaction inhibits the immune response, allowing cancer cells to evade detection and destruction by the immune system. Targeting the PD-1 pathway has revolutionized cancer treatment, leading to the development of immune checkpoint inhibitors that unleash the immune system's ability to fight cancer.
PD-1 Competitive Landscape
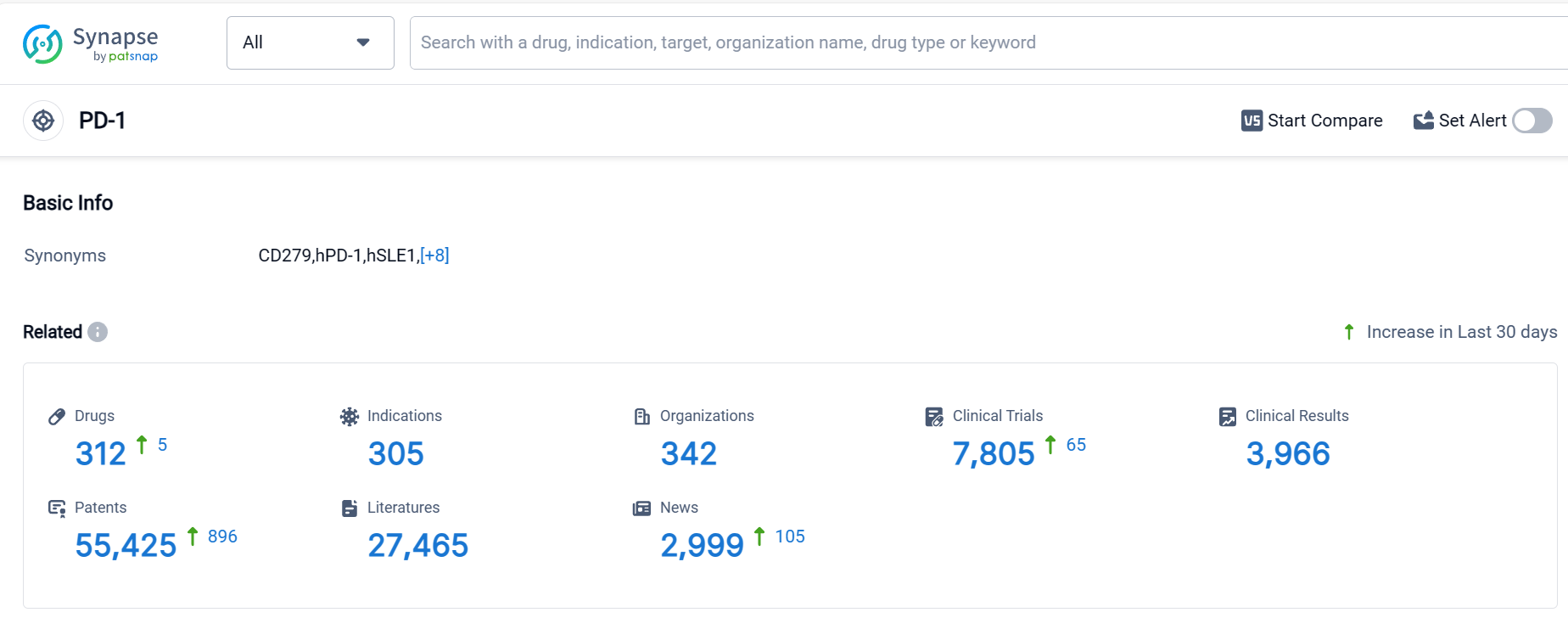
According to Patsnap Synapse, as of 5 Oct 2023, there are a total of 312 PD-1 drugs worldwide, from 342 organizations, covering 305 indications, and conducting 7805 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target PD-1 reveals a competitive landscape with several companies actively involved in research and development. Akeso, Inc., Bristol Myers Squibb Co., and Merck & Co., Inc. are the companies growing fastest under the current target. PD-1 inhibitors have been approved for indications such as Esophageal Squamous Cell Carcinoma, Hodgkin's Lymphoma, Non-Small Cell Lung Cancer, and Melanoma. Monoclonal antibodies and biosimilars are the drug types progressing most rapidly, indicating intense competition. China is developing rapidly in PD-1 research and development, with the highest number of approved drugs and a significant number of drugs in the preclinical stage. Overall, the target PD-1 shows promising potential for the treatment of various cancers, and further research and development are expected in the future.
Key drug:Peresolimab
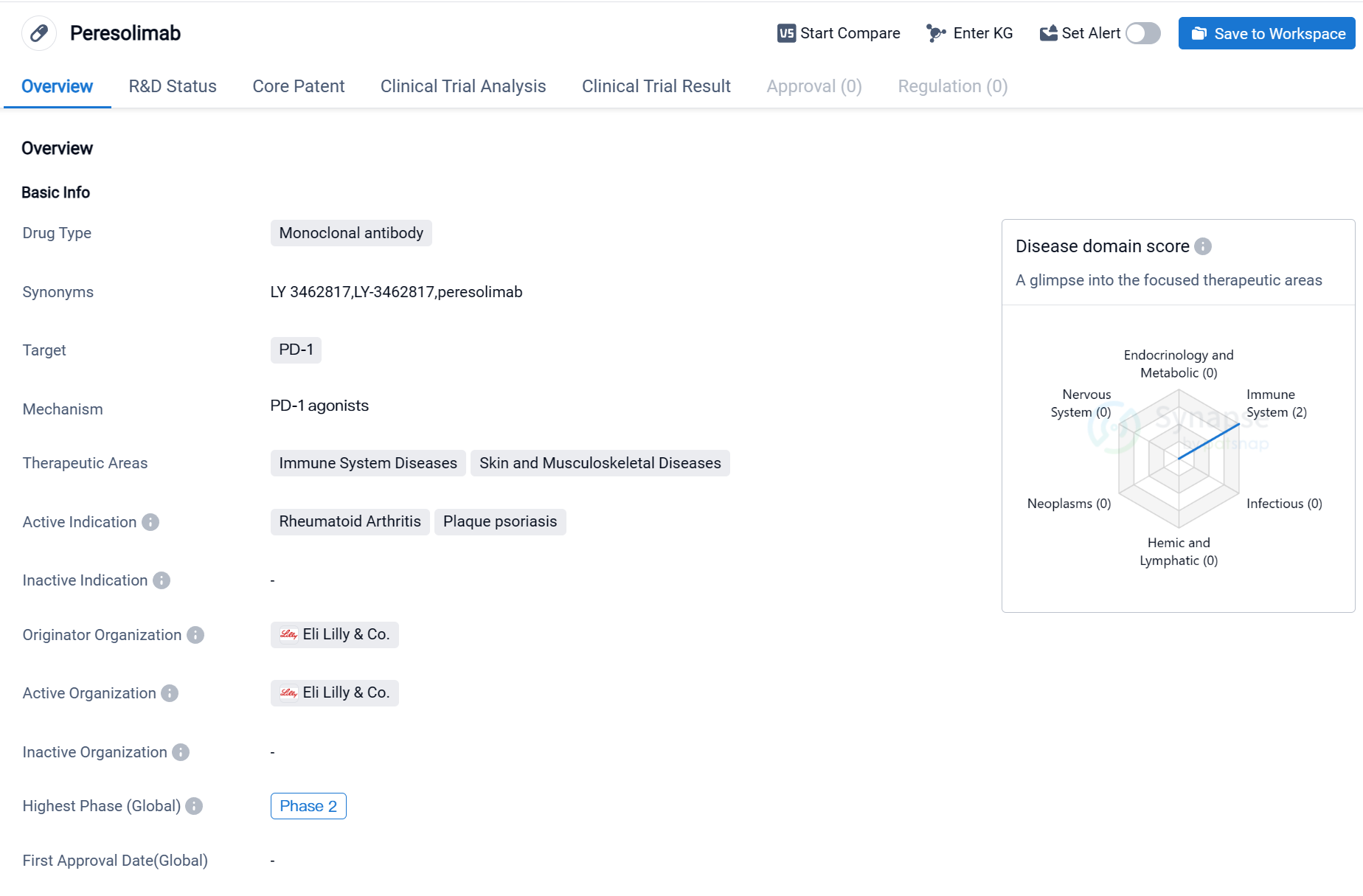
Peresolimab is a monoclonal antibody drug that targets PD-1, a protein involved in regulating the immune system. It is being developed by Eli Lilly & Co., a pharmaceutical company known for its expertise in the field of biomedicine. The drug is currently in Phase 2 of clinical trials, both globally and in China.
The therapeutic areas that Peresolimab aims to address are immune system diseases and skin and musculoskeletal diseases. Specifically, it is being investigated for its potential in treating rheumatoid arthritis and plaque psoriasis. Rheumatoid arthritis is a chronic inflammatory disorder that primarily affects the joints, causing pain, swelling, and stiffness. Plaque psoriasis, on the other hand, is a chronic autoimmune condition characterized by red, raised patches of skin covered with silvery-white scales.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a monoclonal antibody, Peresolimab is designed to bind to PD-1, a receptor found on certain immune cells. By targeting PD-1, the drug aims to modulate the immune response and potentially alleviate the symptoms associated with immune system diseases and skin and musculoskeletal diseases.
The fact that Peresolimab has reached Phase 2 of clinical trials indicates that it has shown promising results in earlier stages of development. Phase 2 trials involve a larger number of participants and are designed to further evaluate the drug's safety and efficacy. If successful, these trials may pave the way for Phase 3 trials, which are typically the final stage before seeking regulatory approval.
It is worth noting that the information provided is objective and based solely on the given data. No subjective interpretations or fictional data have been added. The summary provides a concise overview of Peresolimab, its drug type, targets, therapeutic areas, active indications, originator organization, and the current phase of development both globally and in China.