Analysis on the Research Progress of p53 inhibitors
p53 inhibitors are a class of chemical compounds that target and inhibit the function of the p53 protein. The p53 protein, also known as the tumor protein 53, is a critical regulator of cell cycle progression, DNA repair, and apoptosis (programmed cell death). It acts as a tumor suppressor by preventing the proliferation of cells with damaged DNA or mutations that could lead to cancer development. In normal conditions, the p53 protein is tightly regulated and maintained at low levels in the cell. However, in response to various stress signals such as DNA damage, oncogene activation, or hypoxia, the levels and activity of p53 can increase. Activated p53 induces cell cycle arrest, allowing time for DNA repair, or triggers apoptosis if the DNA damage is irreparable. This mechanism helps to maintain genomic stability and prevent the survival and propagation of cells with potentially harmful mutations.
p53 inhibitors interfere with the normal function of the p53 protein and are primarily used as research tools to study the biological effects of p53 inactivation. By inhibiting p53, these chemicals allow researchers to investigate the consequences of p53 loss or reduced activity in cellular processes and disease models. There are several types of p53 inhibitors, and they can target different aspects of p53 function. One common approach is to disrupt the interaction between p53 and its negative regulator, MDM2 (mouse double minute 2). MDM2 binds to p53 and promotes its degradation, thereby regulating its levels in the cell. Inhibitors that interfere with the p53-MDM2 interaction prevent MDM2 from tagging p53 for degradation, leading to increased levels and activity of p53. Overall, the p53 pathway is highly complex and involves numerous interactions with other proteins and regulatory mechanisms. Many p53 inhibitors bind directly to the p53 protein and disrupt it's interaction with other proteins involved in p53 signaling pathways. These inhibitors can also interfere with the ability of p53 to activate downstream genes involved in cell cycle arrest or apoptosis.
p53 is a crucial protein that plays a vital role in maintaining the integrity of the human body. It acts as a tumor suppressor by regulating cell division and preventing the formation of cancerous cells. p53 monitors DNA damage and initiates repair mechanisms, halting cell division if the damage is irreparable. Additionally, it promotes programmed cell death (apoptosis) to eliminate cells with severe DNA damage. p53 also regulates various cellular processes, including metabolism, angiogenesis, and immune response. Its significance in preventing cancer and maintaining genomic stability makes p53 an essential protein in the human body.
p53 Competitive Landscape
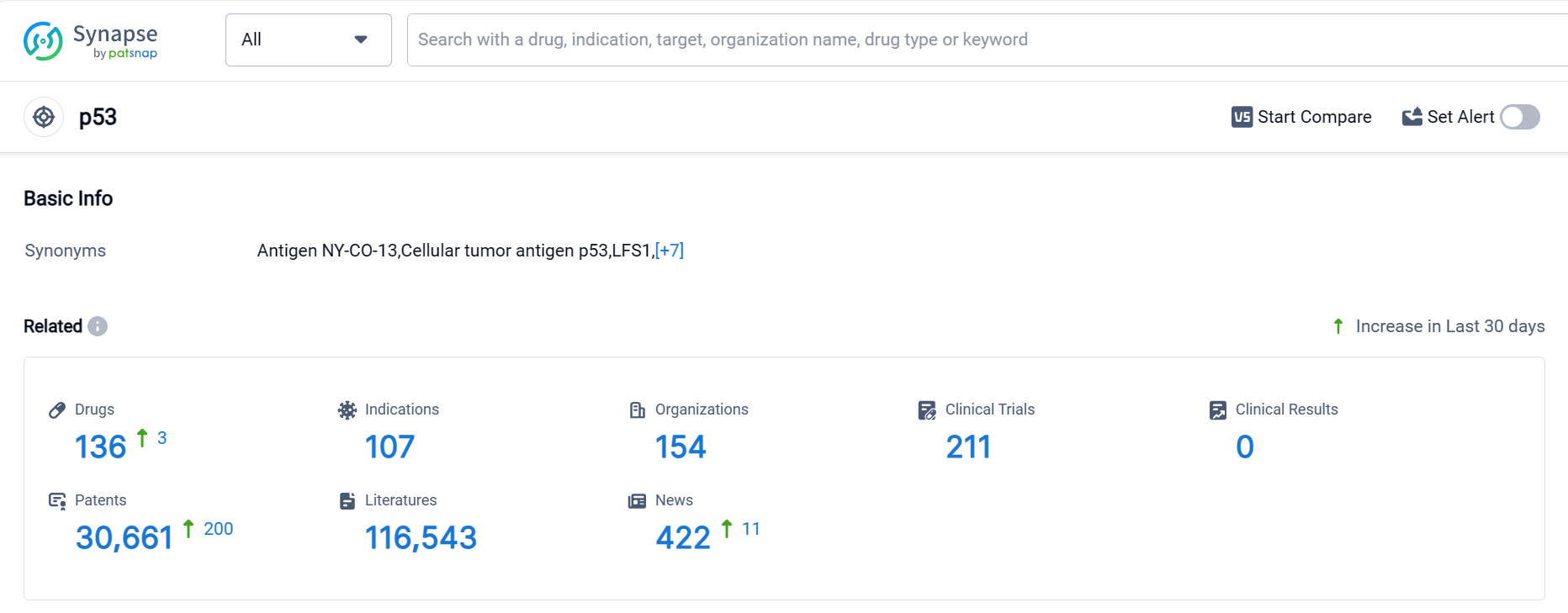
According to Patsnap Synapse, as of 5 Oct 2023, there are a total of 136 p53 drugs worldwide, from 154 organizations, covering 107 indications, and conducting 211 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the current competitive landscape and future development of target p53 reveals a diverse and dynamic pharmaceutical industry. Aprea Therapeutics, Inc. is a key player in the development of drugs targeting p53, with a strong focus on advancing their drugs through clinical trials. The indications for target p53 cover a wide range of diseases and conditions, indicating its potential in various therapeutic areas. The presence of multiple drug types and the intense competition around biosimilars suggest a diverse and innovative approach to targeting p53. China, the United States, and other countries/locations are actively involved in the development of drugs targeting p53, with China showing significant progress. Overall, the future development of target p53 holds promise for advancements in the treatment of various diseases and conditions.
Key drug:Teprasiran
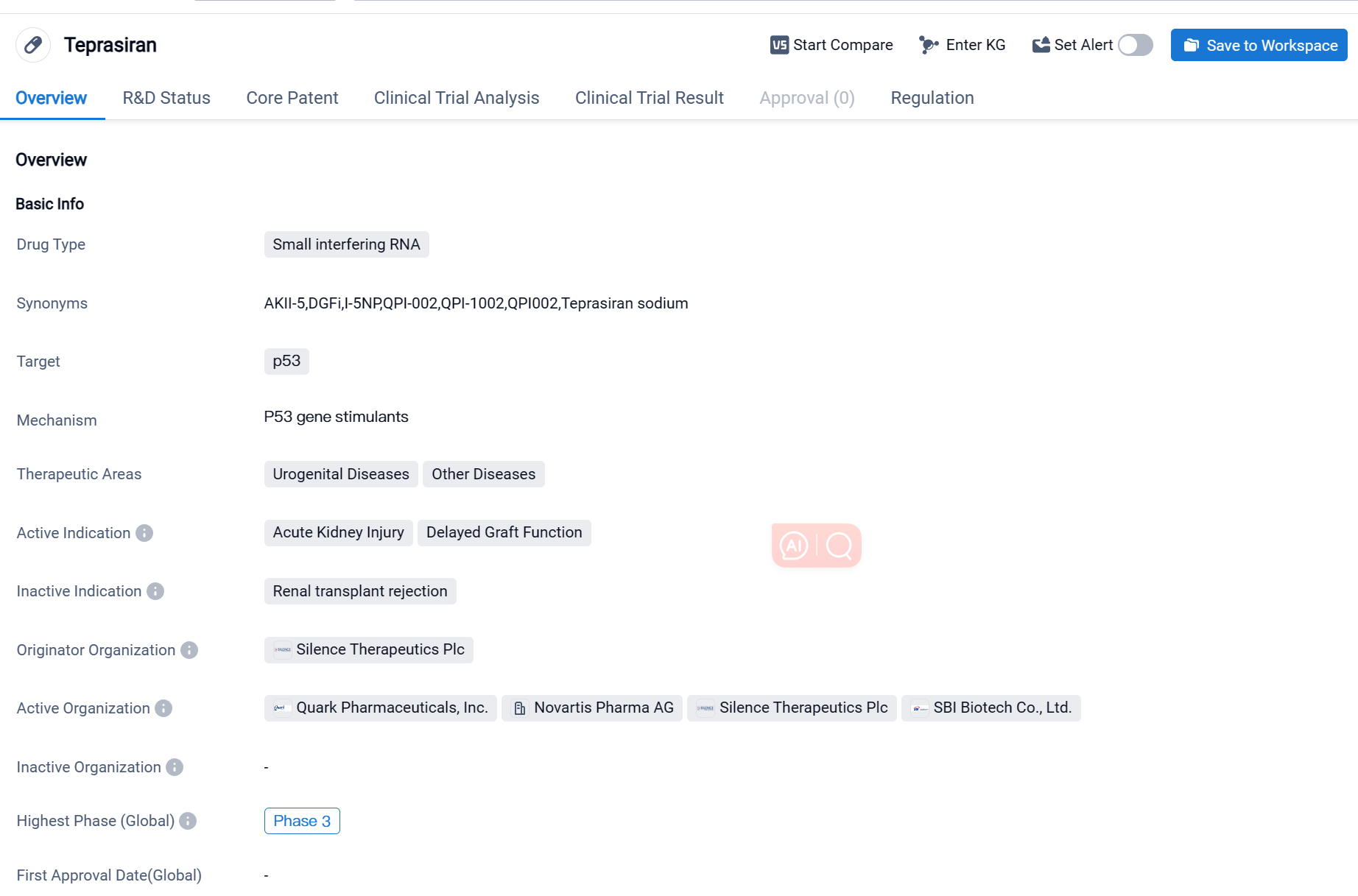
Teprasiran is a small interfering RNA (siRNA) drug that targets the p53 protein. It is being developed by Silence Therapeutics Plc, a pharmaceutical company specializing in RNA interference (RNAi) therapeutics. The drug is currently in Phase 3, which is the final stage of clinical trials before seeking regulatory approval.
Teprasiran is primarily being investigated for its potential use in treating urogenital diseases and other diseases. Specifically, it is being studied for its efficacy in treating acute kidney injury and delayed graft function. These conditions are characterized by damage to the kidneys, which can lead to impaired kidney function and potentially life-threatening complications.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
One notable aspect of Teprasiran is its regulatory status as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. The designation provides certain incentives and benefits to the drug developer, such as extended market exclusivity and financial support for clinical trials.
siRNA drugs like Teprasiran work by interfering with the expression of specific genes, in this case, the p53 gene. The p53 protein is known as the "guardian of the genome" and plays a crucial role in regulating cell growth and preventing the formation of tumors. By targeting p53, Teprasiran aims to modulate its activity and potentially provide therapeutic benefits in the treatment of urogenital diseases and other conditions.
As Teprasiran is currently in Phase 3, it is undergoing rigorous testing to evaluate its safety and efficacy in a larger patient population. The results of these trials will determine whether the drug can proceed to regulatory approval and ultimately be made available to patients.
In summary, Teprasiran is a siRNA drug developed by Silence Therapeutics Plc that targets the p53 protein. It is being investigated for its potential use in treating urogenital diseases and other conditions, specifically acute kidney injury and delayed graft function. With its orphan drug designation and current Phase 3 status, Teprasiran holds promise as a potential therapeutic option for patients in need.