Apogee Therapeutics Initiates Phase 1 Trial of APG990, a Long-Acting OX40L Antibody
Apogee Therapeutics, Inc. (Nasdaq: APGE), a biotech company in the clinical stages focusing on the development of innovative biologics aimed at achieving improved efficacy and dosing in major inflammatory and immunology (I&I) markets such as atopic dermatitis (AD), asthma, chronic obstructive pulmonary disease (COPD), and other I&I conditions, announced today the commencement of dosing in its inaugural clinical trial for APG990. This novel subcutaneous (SQ) extended half-life monoclonal antibody targets OX40L and is being primarily developed as a therapy for individuals with AD.
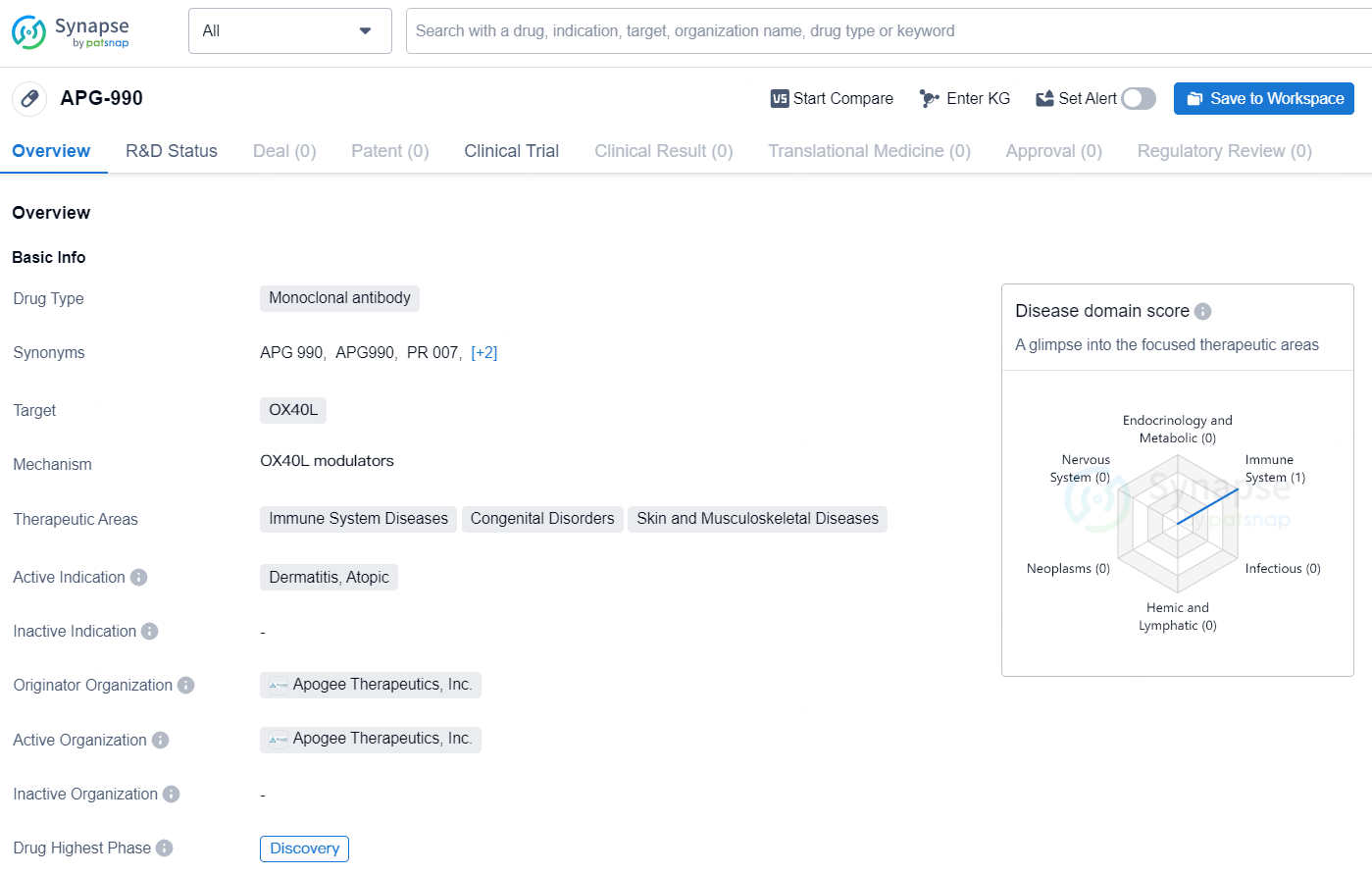
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The initiation of the APG990 Phase 1 clinical trial marks a significant milestone for Apogee as we strive for excellence in our operations. In the past year, we have successfully moved three of our programs into clinical trial phases, offering the potential for new treatments to patients with prevalent I&I conditions," stated Michael Henderson, M.D., Chief Executive Officer of Apogee. "Our initial focus with APG990 is on AD, and we believe the combination of IL-13's (APG777) active mechanism with OX40L (APG990) could extend patient reach and deliver superior efficacy and dosing within its class. This strategy highlights our unwavering commitment to advancing innovative treatment options for AD. We aim to surpass mere adequacy."
The Phase 1 clinical trial of APG990 is structured as a double-blind, placebo-controlled, first-in-human study, involving single-ascending doses administered to healthy volunteers. This trial is set to assess the safety, tolerability, and pharmacokinetics (PK) of APG990, with an anticipated enrollment of roughly 40 healthy adults across 5 cohorts. Interim data from this trial is expected in 2025.
Subject to favorable outcomes from the Phase 1 clinical trial and subsequent submission of an Investigational New Drug application or its foreign counterpart, the company intends to begin a Phase 1 clinical trial combining APG777 and APG990. This novel approach seeks to test the combination's effectiveness in providing sustained inhibition of Type 2 inflammation through APG777's targeted IL-13 inhibition, alongside broader inhibition of Types 1-3 inflammation via APG990's OX40L inhibition. The initial combination trial is projected to commence in 2025.
“The advancement of the APG990 program signifies a critical step in our mission to improve treatment options for AD patients,” noted Carl Dambkowski, M.D., Chief Medical Officer of Apogee. "AD exhibits heterogeneity, with Type 2 inflammation being the primary pathway and varying degrees of involvement from Type 1 and Type 3 inflammation. Currently, available therapies focus on the Type 2 pathway. However, OX40L has shown clinical validation for inhibiting all three inflammation pathways, potentially enhancing treatment capabilities. We are investigating APG777 and APG990, our extended half-life mAbs, to provide more robust and extensive responses, aiming for improved overall results and optimal dosing for patients with AD and other I&I diseases.”
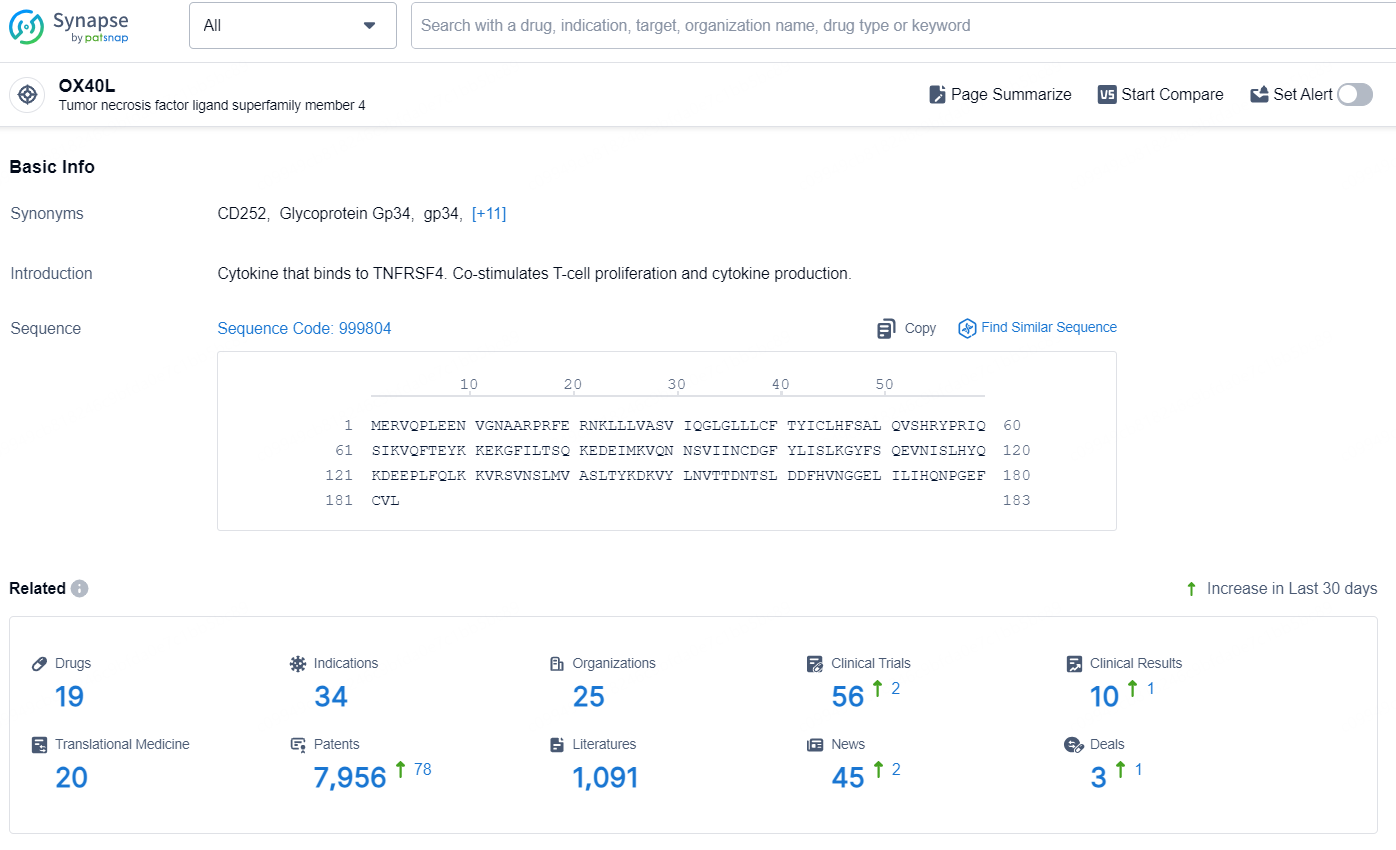
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 22, 2024, there are 19 investigational drugs for the OX40L target, including 34 indications, 25 R&D institutions involved, with related clinical trials reaching 56, and as many as 7956 patents.
APG990 is a novel, SQ half-life extended mAb targeting OX40L, initially being developed for AD. OX40L is located further upstream in the inflammatory pathway than IL-13 or IL-4Rα and targeting it could potentially have broader impact on the inflammatory cascade by inhibiting Type 1, Type 2 and Type 3 pathways. AD is a heterogeneous disease and varies by age, severity and ethnicity. With current approved biologics only targeting two mechanisms of action (IL-13 and IL4Rα) in AD, OX40L could represent another therapeutic option for patients, especially the portion of patients who do not benefit from currently available treatments.