Apollo Therapeutics Licenses Sunshine Lake Pharma's Dual Receptor Agonist
Apollo Therapeutics Group Limited (“Apollo”), a biopharmaceutical company managing a portfolio of products, has established an exclusive licensing partnership with Sunshine Lake Pharma Co., Ltd., an innovative pharmaceutical organization and a part of HEC Group, recognized as one of China's leading 500 private enterprises. This agreement focuses on the development of APL-18881 (HEC88473). According to the agreement, Sunshine Lake will maintain the rights for development, production, and commercialization within China, while granting Apollo similar rights for the rest of the globe concerning all existing and future therapeutic applications. Sunshine Lake will receive an initial payment of $12 million and could earn up to $926 million in milestone payments associated with development, regulatory approvals, and commercialization. These milestone payments are linked to achieving specific annual sales targets in major markets. Furthermore, Sunshine Lake is eligible to earn tiered royalties that range from low double digits to high single digits based on net sales.
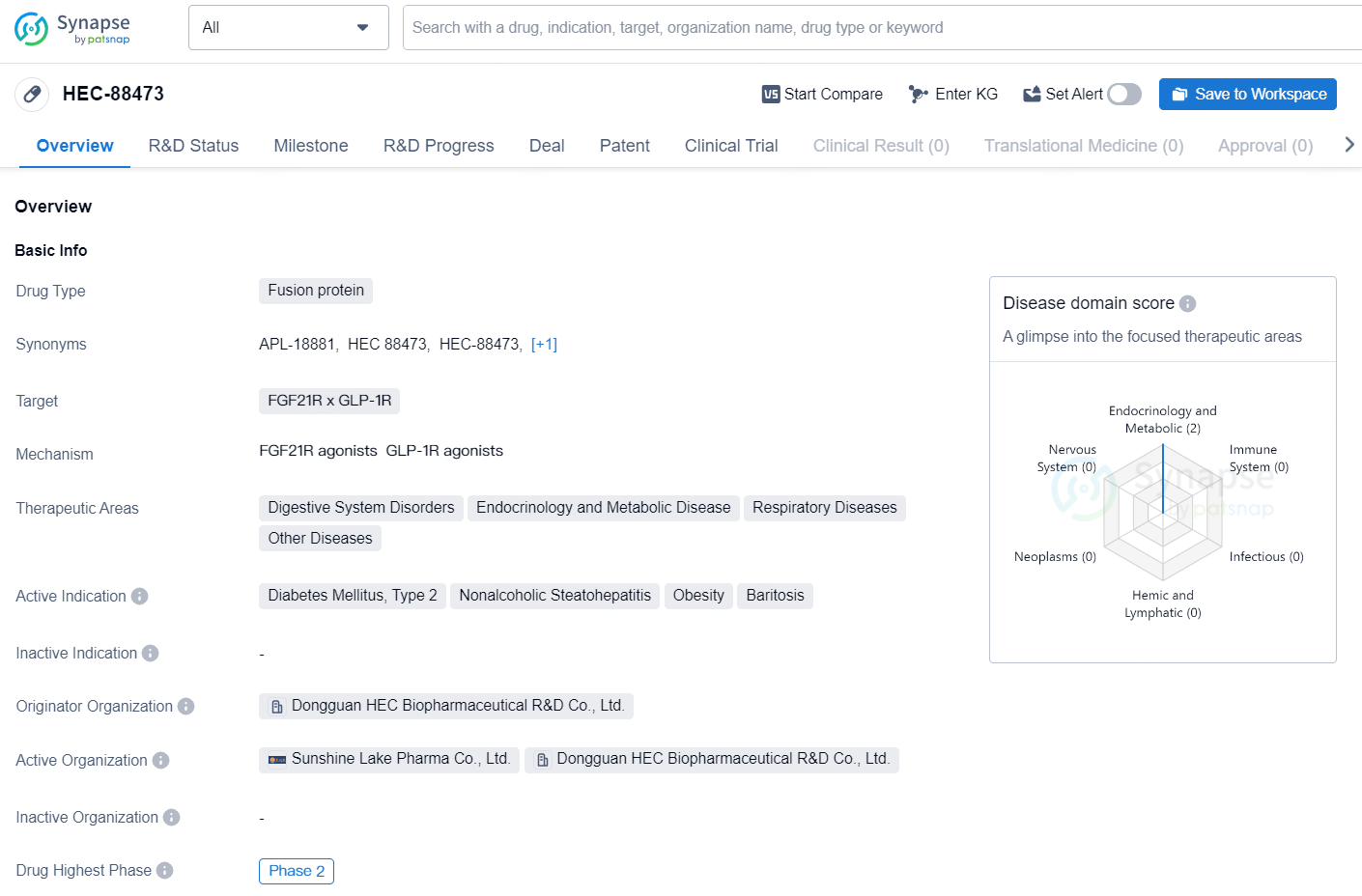
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Apollo is creating a comprehensive and varied portfolio of innovative drug candidates, with APL-18881 (HEC88473) representing its fifth clinical development initiative. In addition to identifying and advancing new therapeutics derived from fundamental scientific breakthroughs at partner universities, Apollo also pursues the in-licensing or acquisition of clinical-stage projects, adhering to rigorous selection standards. These standards are focused on leveraging unique insights and synergies from its discovery work, human genetics, and other preclinical research. APL-18881 (HEC88473) is currently supported by an open US Investigational New Drug Application (IND), and Apollo plans to move forward with clinical trials targeting various potential indications within the cardiometabolic, liver, and related disease sectors, which have not yet been publicly disclosed.
Sunshine Lake has a strong commitment to advancing innovative drug development in fields such as anti-infection, oncology, and chronic metabolic disorders, and it has built a solid pipeline featuring numerous programs at mid-to-late stages of clinical trials. While primarily concentrating on domestic development and commercialization for its novel drugs within China, Sunshine Lake is also actively seeking to broaden its reach into international markets. The partnership with Apollo on the APL-18881 (HEC88473) project represents Sunshine Lake's inaugural foray into international collaboration for novel biologics, highlighting the company's dedication to expanding its global presence.
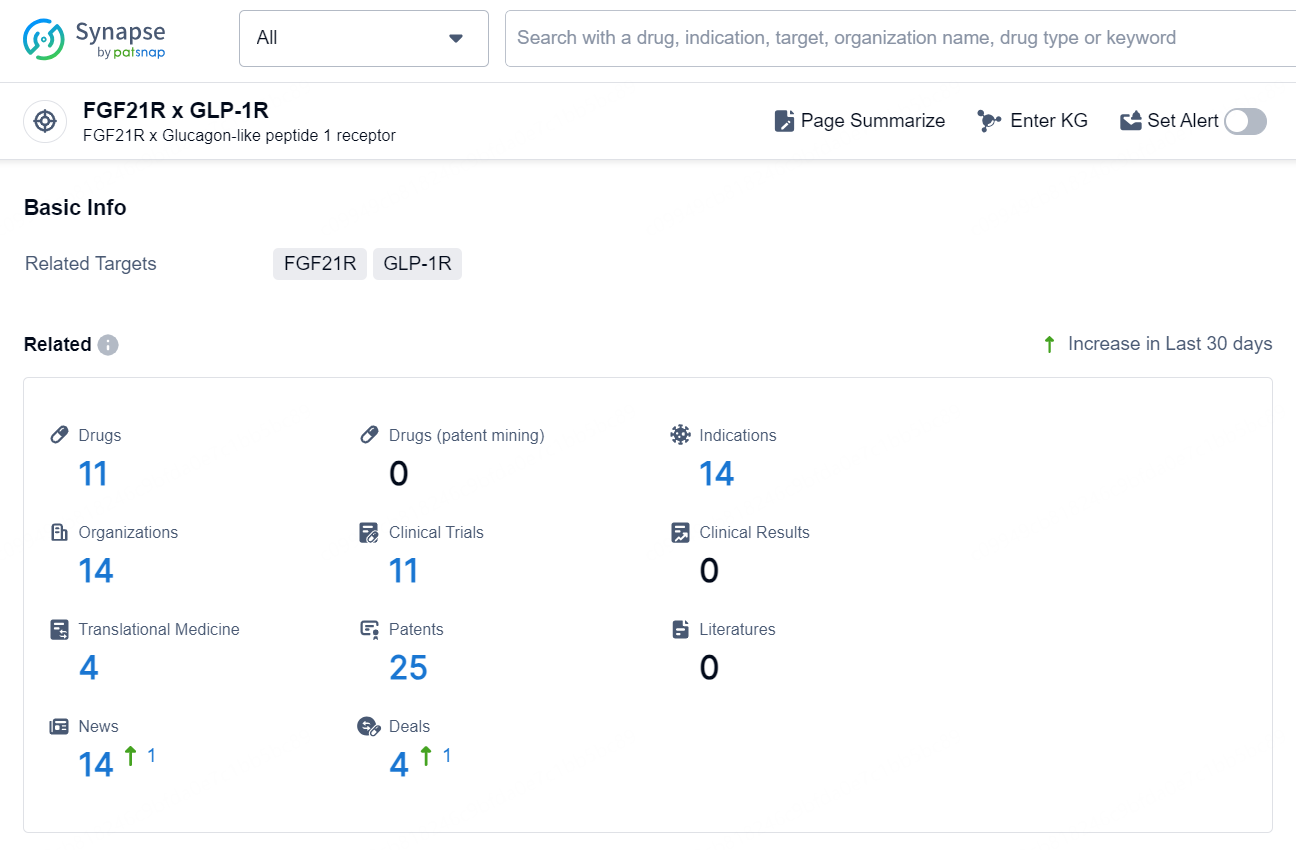
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 13, 2024, there are 11 investigational drugs for the FGF21R x GLP-1R target, including 14 indications, 14 R&D institutions involved, with related clinical trials reaching 11, and as many as 25 patents.
HEC-88473 is a fusion protein drug developed by Dongguan HEC Biopharmaceutical R&D Co., Ltd. The drug targets FGF21R x GLP-1R and is indicated for therapeutic areas such as Digestive System Disorders, Endocrinology and Metabolic Disease, Respiratory Diseases, and Other Diseases. The active indications for HEC-88473 include Diabetes Mellitus, Type 2, Nonalcoholic Steatohepatitis, Obesity, and Baritosis.