Apretude (Cabotegravir) Shows Over 99% Effectiveness in Real-World Studies, Reports ViiV Healthcare
ViiV Healthcare, a global leader in HIV treatment and primarily owned by GSK, with Pfizer and Shionogi as its stakeholders, has revealed new real-world evidence and implementation findings demonstrating the efficacy, adherence rates, and improvements in quality of life associated with Apretude (cabotegravir long-acting (CAB LA)) for HIV pre-exposure prophylaxis (PrEP). These findings are set to be presented at IDWeek 2024, taking place in Los Angeles, California from October 16 to 19.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Results from two real-world evidence studies (the OPERA and Trio Health cohorts) indicated an effectiveness of over 99% for CAB LA as a PrEP method among nearly 1,300 participants. The PILLAR implementation study demonstrated reductions in stigma and anxiety levels for the 200 individuals utilizing this long-acting injectable PrEP option.
Harmony P. Garges, M.D., MPH, Chief Medical Officer at ViiV Healthcare, stated: “The results shared at IDWeek 2024 reinforce the consistent and robust effectiveness of Apretude for individuals in real-world settings, beyond the confines of clinical trials. The findings from the OPERA and Trio cohorts contribute to the expanding evidence base collected over the past three years, confirming that CAB LA for PrEP is a profoundly effective strategy for HIV prevention. We hold the view that long-acting treatments possess the capacity to significantly enhance the adoption of PrEP among a wide spectrum of individuals who may benefit, which is essential for eradicating the HIV epidemic.”
Data from the Trio Health cohort illustrated the real-world efficacy of CAB LA for PrEP, with no instances of HIV acquisition reported during the follow-up period.
Recent insights from the Trio Health cohort emphasized its effectiveness in preventing HIV infection and maintaining adherence among 474 individuals in the U.S. The evaluation revealed a varied demographic of cisgender and transgender individuals initiating CAB LA for PrEP based on electronic health records collected from December 2021 to January 2024.
Results indicated that there were no recorded HIV diagnoses among participants receiving CAB LA for PrEP throughout the follow-up duration. At the time of analysis, 83% of participants remained engaged with CAB LA for PrEP injections, with most injections administered punctually by new users. Among the 396 individuals receiving continued injections, 33% faced delays, with a median of one delayed injection and a median delay period of 12 days [IQR: 3-29]. Adherence rates for CAB LA for PrEP were notably high, with just 3% of participants reporting a missed injection.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
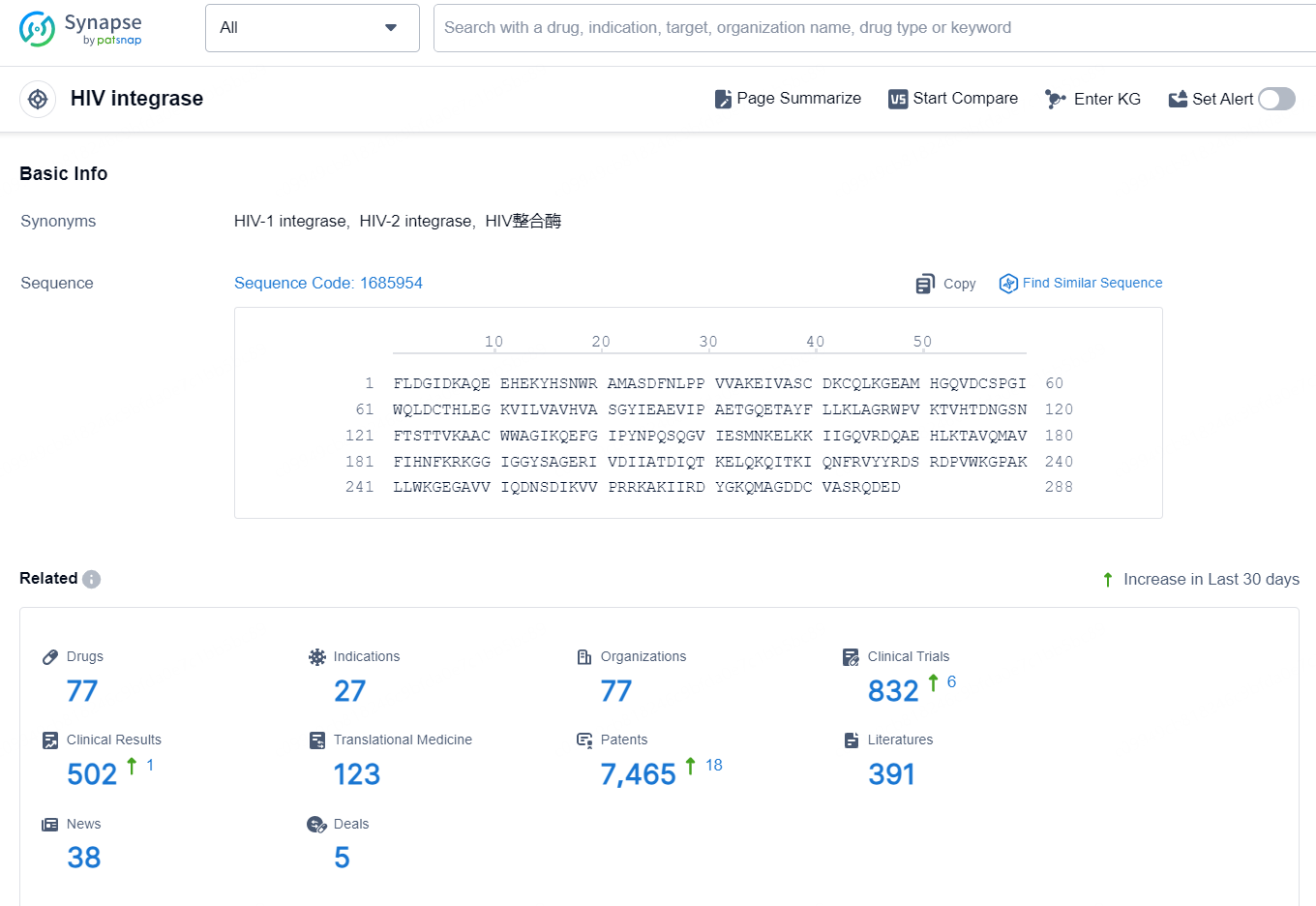
According to the data provided by the Synapse Database, As of October 18, 2024, there are 77 investigational drugs for the HIV integrase target, including 27 indications, 77 R&D institution involved, with related clinical trial reaching 832, and as many as 7465 patents.
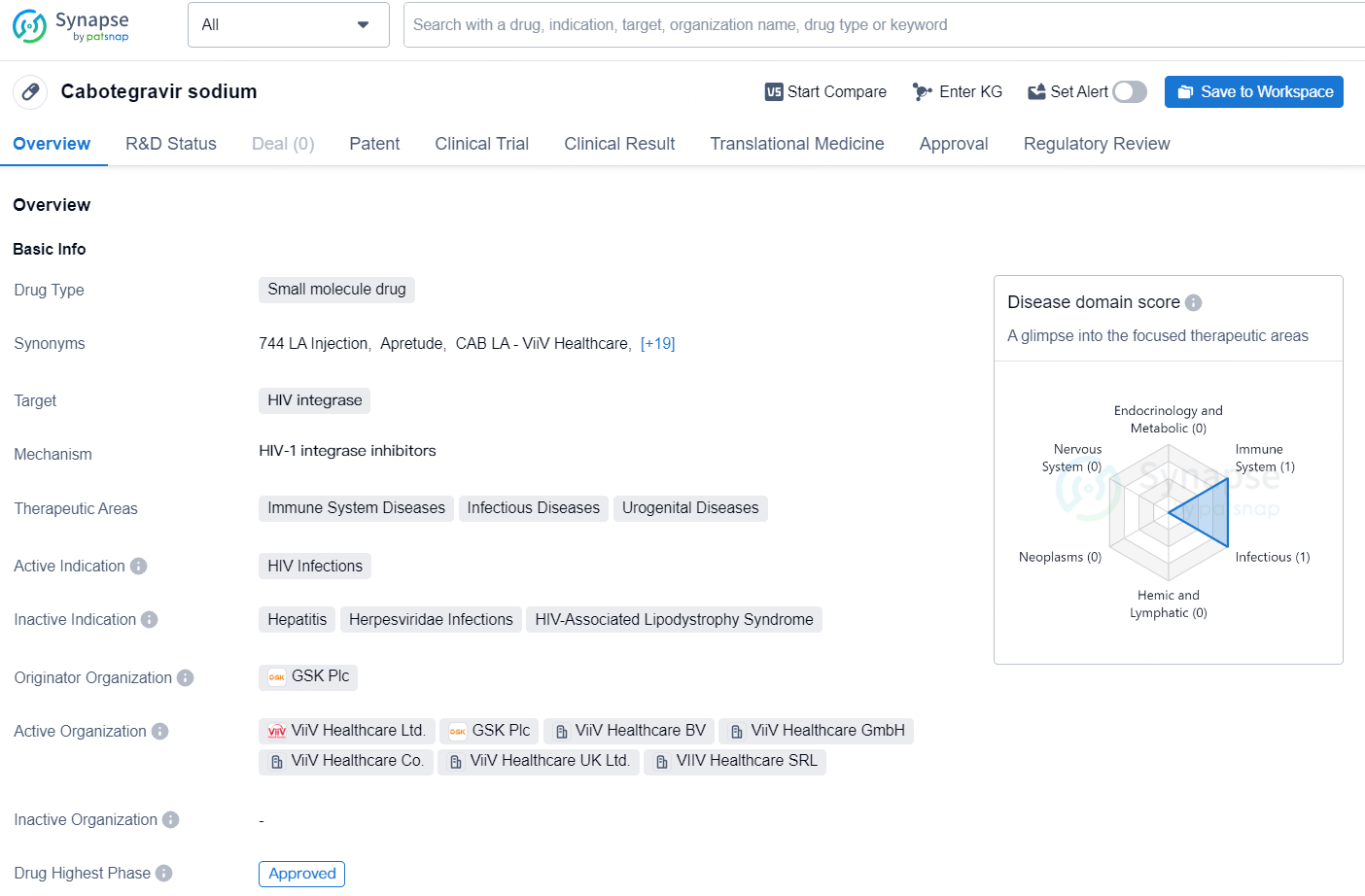
Cabotegravir sodium is a small molecule drug that targets HIV integrase and is primarily used for the treatment of HIV Infections. It falls within the therapeutic areas of Immune System Diseases, Infectious Diseases, and Urogenital Diseases. The drug was developed by GSK Plc, a global pharmaceutical company, and has achieved approval in both the global market and specifically in China.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!