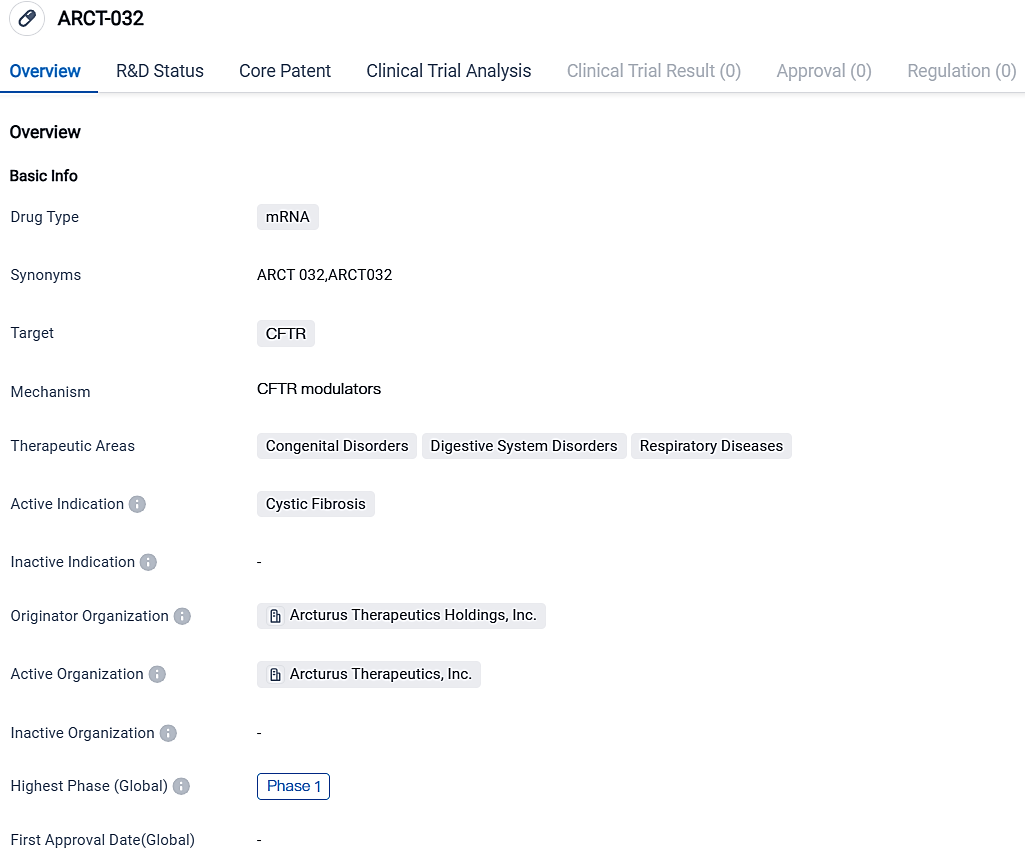
Arcturus Therapeutics and the Cystic Fibrosis Foundation are collaborating on the production of ARCT-032, a possible mRNA treatment for Cystic Fibrosis
Arcturus Therapeutics Holdings Inc., a worldwide corporation in the later stages of clinical trials for messenger RNA drugs, primarily concentrating on creating vaccines for infectious diseases and exploring possibilities in the field of rare liver and respiratory diseases, has made public that their current contract with the Cystic Fibrosis Foundation has been extended.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The CF Foundation has pledged to boost its funding commitment to $25 million to promote ARCT-032, an innovative messenger RNA therapeutic option developed using Arcturus' LUNAR® distribution technology. This commitment expands upon a previous research initiative launched in 2017, which has facilitated the progression of ARCT-032 into current clinical evaluations.
"We are excited to continue our fruitful partnership with the Cystic Fibrosis Foundation, and we appreciate their significant financial assistance," expressed Pad Chivukula, Ph.D., Chief Scientific Officer at Arcturus.
Dr. Chivukula further added, "ARCT-032 exhibits the capacity to produce a fully operational CFTR protein in the lung, directly targeting the underlying cause of cystic fibrosis. The resources and valuable know-how offered by the CF Foundation will be instrumental in bolstering the ongoing clinical development of ARCT-032, incorporating the finalization of an impending Phase 1b study intended for adults affected by cystic fibrosis."
ARCT-032 is comprised of mRNA that produces CFTR, encapsulated by lipid nanoparticles by employing the LUNAR delivery system, optimized specifically for pulmonary administration. ARCT-032 can function regardless of associated disease mutations and holds the promise to offer clinical advantages to a broad population with CF, including those not benefiting from presently approved CFTR modulators. The program has concluded the medication dosage in a Phase 1 trial involving healthy participants.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
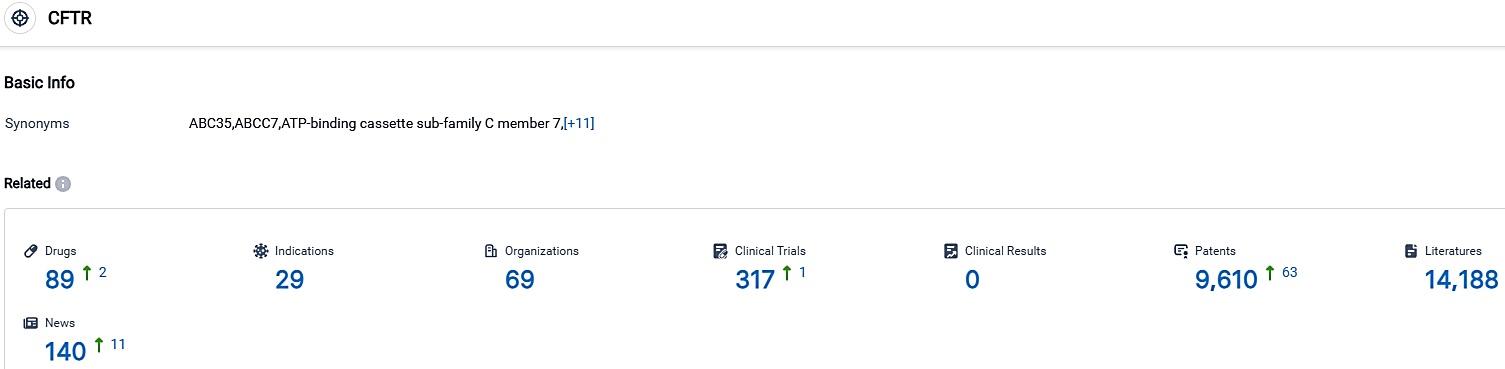
According to the data provided by the Synapse Database, As of October 2, 2023, there are 89 investigational drugs for the CFTR target, including 29 indications,69 R&D institutions involved, with related clinical trials reaching 317,and as many as 9610 patents.
ARCT-032 will deploy Arcturus' LUNAR® lipid-based aerosolized system for conveying CFTR mRNA to the lungs. Presenting a functioning version of the CFTR mRNA in the lungs of those suffering from CF, may potentially revitalize CFTR functionality and alleviate the subsequent impacts leading to evolving lung disease. The ARCT-032 initiative is backed by preclinical evidence in rodents, ferrets, and primates. In addition to this, it has shown signs of restored expression and functionality in human bronchial epithelial cells.