ROCK2 inhibitors help to restore immune balance
ROCK, also known as Rho-associated coiled-coil kinases, is a class of serine/threonine protein kinases that play an important role in various common cellular functions such as cell proliferation, cell apoptosis, and gene expression.
ROCK is a downstream effector of RhoA. There are two subtypes, ROCK1 and ROCK2. ROCK1 is mainly expressed in non-neural tissues such as lungs, kidneys, and skeletal muscles, while ROCK2 is mainly expressed in the central nervous system, including hippocampal pyramidal neurons, the cerebral cortex, and cerebellar Purkinje cells. The Rho/ROCK signaling pathway is an important signaling transduction system involved in cell growth, differentiation, migration, and development.
For example, in fibrotic diseases, the response of the myocyte to injury involves the reorganization of the actin cytoskeleton of various cells, and the assembly of actin filaments and the contraction of actin myosin are guided and regulated by the ROCK family proteins (including ROCK1 and ROCK2). Similarly, in chronic graft-versus-host disease (cGVHD), a complication following allogeneic hematopoietic stem cell transplantation (allo-HSCT), the transplanted immune cells attack the host's healthy cells, causing inflammatory reactions and fibrosis in multiple organs and tissues. The inhibition of ROCK2 kinase is expected to help restore immune balance.
ROCK2 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 25 Sep 2023, there are a total of 25 ROCK2 drugs worldwide, from 36 organizations, covering 45 indications, and conducting 60 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.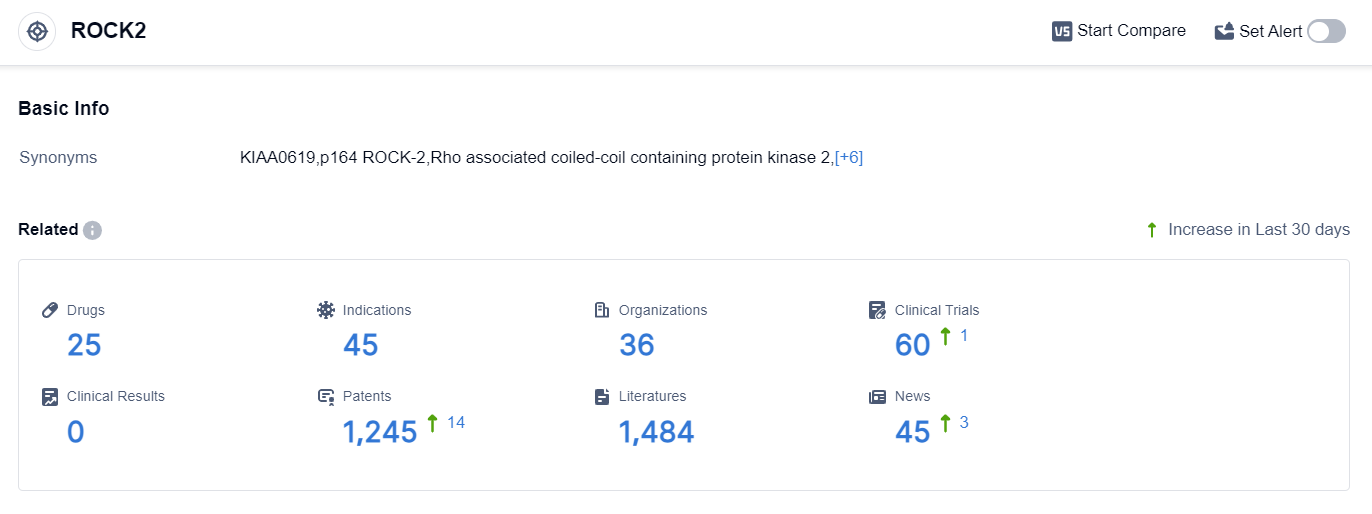
The analysis of the current competitive landscape of target ROCK2 reveals that Sanofi is the leading company with the highest stage of development. The indication analysis shows that drugs under this target have been approved for chronic graft-versus-host disease and glaucoma, open-angle. Small molecule drugs are progressing rapidly, indicating intense competition.
The countries/locations developing fastest include the United States, China, Japan, South Korea, and various countries in the European Union. The future development of target ROCK2 holds potential for advancements in the treatment of various indications, including idiopathic pulmonary fibrosis, ocular hypertension, and rheumatoid arthritis.
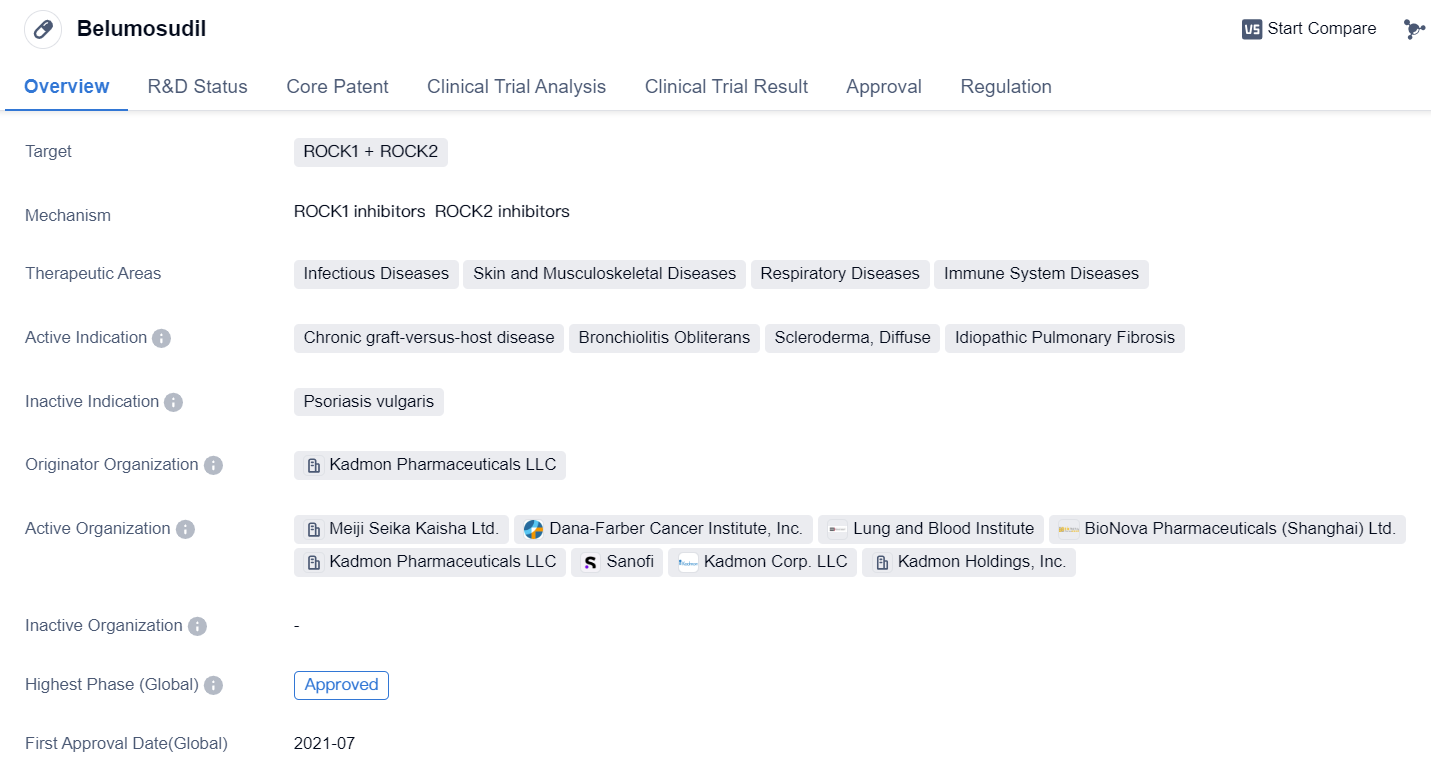
Approved ROCK2 Inhibitors for market launch: Belumosudil
Belumosudilis a small molecule drug that targets ROCK1 and ROCK2. It falls under the therapeutic areas of Infectious Diseases, Skin and Musculoskeletal Diseases, Respiratory Diseases, and Immune System Diseases. The drug is indicated for the treatment of Chronic graft-versus-host disease, Bronchiolitis Obliterans, Scleroderma, Diffuse, and Idiopathic Pulmonary Fibrosis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Belumosudil was developed by Kadmon Pharmaceuticals LLC, an originator organization in the pharmaceutical industry. It has reached the highest phase of development, with approvals in both the global market and China. The drug received its first approval in the United States in July 2021.
In terms of regulations, Belumosudil has undergone Priority Review, indicating its potential to address unmet medical needs. It has also been designated as an Orphan Drug, which signifies its intended use for rare diseases. Additionally, the drug has been granted Breakthrough Therapy status, highlighting its promising clinical results and potential to provide significant benefits over existing treatments.
The approval of Belumosudil marks a significant milestone in the field of biomedicine. Chronic graft-versus-host disease, Bronchiolitis Obliterans, Scleroderma, Diffuse, and Idiopathic Pulmonary Fibrosis are complex and challenging conditions that affect various organ systems. The targeted inhibition of ROCK1 and ROCK2 by Belumosudil offers a novel approach to managing these diseases.
Overall, Belumosudil's approval represents a significant advancement in the treatment options available for patients with Chronic graft-versus-host disease, Bronchiolitis Obliterans, Scleroderma, Diffuse, and Idiopathic Pulmonary Fibrosis. Its unique mechanism of action and broad therapeutic areas make it a promising addition to the pharmaceutical industry's arsenal in combating these challenging diseases.
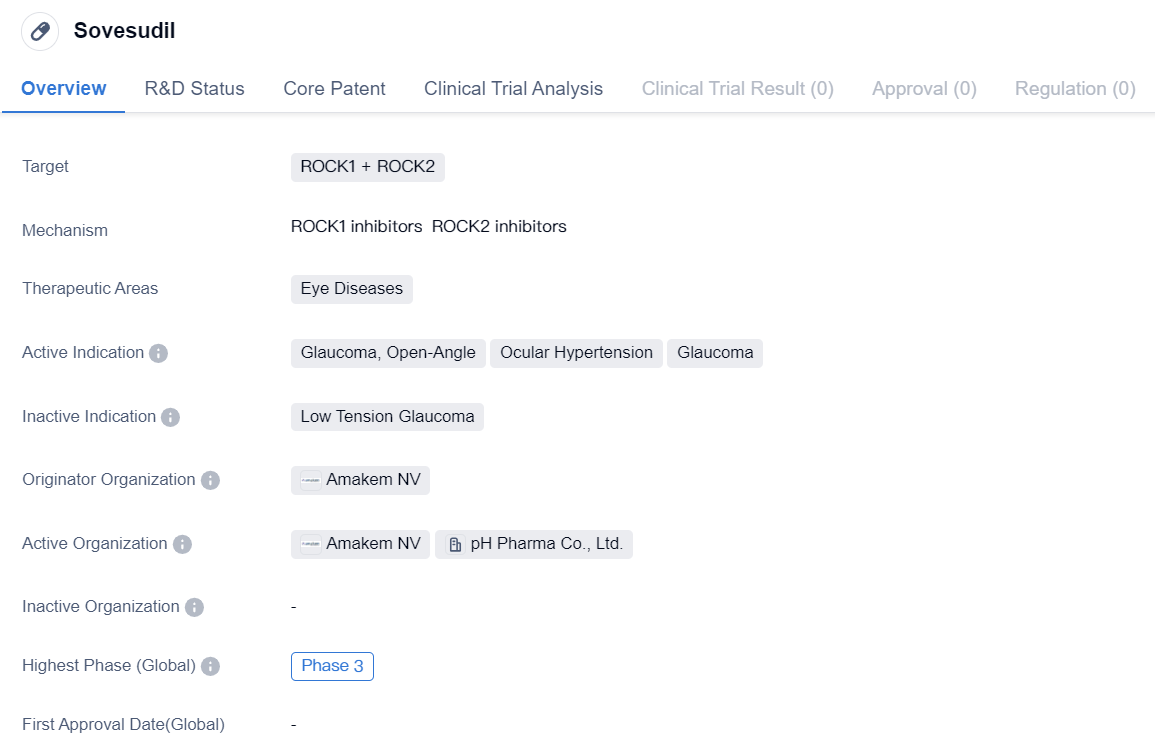
Entering into Phase III Clinical Trials ROCK2 Inhibitors: Sovesudil
Sovesudil is a small molecule drug that targets ROCK1 and ROCK2, and it is primarily used in the treatment of eye diseases. The active indications for Sovesudil include glaucoma, open-angle, ocular hypertension, and glaucoma. The drug is currently in Phase 3 of development, indicating that it has progressed through earlier stages of clinical trials and is now being tested on a larger scale.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Sovesudil is developed by Amakem NV, an originator organization in the pharmaceutical industry. As a small molecule drug, it is designed to interact with specific targets, in this case, ROCK1 and ROCK2. These targets are believed to play a role in the pathogenesis of eye diseases, particularly glaucoma. By targeting these specific proteins, Sovesudil aims to modulate their activity and potentially provide therapeutic benefits for patients with glaucoma and ocular hypertension.
Glaucoma is a chronic eye condition characterized by increased intraocular pressure, which can lead to optic nerve damage and vision loss if left untreated. Open-angle glaucoma is the most common form of the disease, accounting for the majority of glaucoma cases. Ocular hypertension refers to elevated intraocular pressure without any signs of optic nerve damage or vision loss. By targeting ROCK1 and ROCK2, Sovesudil aims to reduce intraocular pressure and potentially slow down or prevent the progression of glaucoma.
The fact that Sovesudil is in Phase 3 of its development is significant. Phase 3 trials are typically large-scale studies involving a larger number of patients to further evaluate the drug's safety and efficacy. This phase is crucial in determining whether the drug can be approved for commercialization and made available to patients. If Sovesudil successfully completes Phase 3 trials and demonstrates positive results, it may have the potential to become a valuable treatment option for individuals with glaucoma and ocular hypertension.
In summary, Sovesudil is a small molecule drug developed by Amakem NV that targets ROCK1 and ROCK2. It is being developed for the treatment of eye diseases, specifically glaucoma, open-angle, ocular hypertension, and glaucoma. Currently in Phase 3 of its development, Sovesudil is undergoing large-scale clinical trials to assess its safety and efficacy. If successful, it may offer a promising therapeutic option for patients suffering from glaucoma and ocular hypertension.