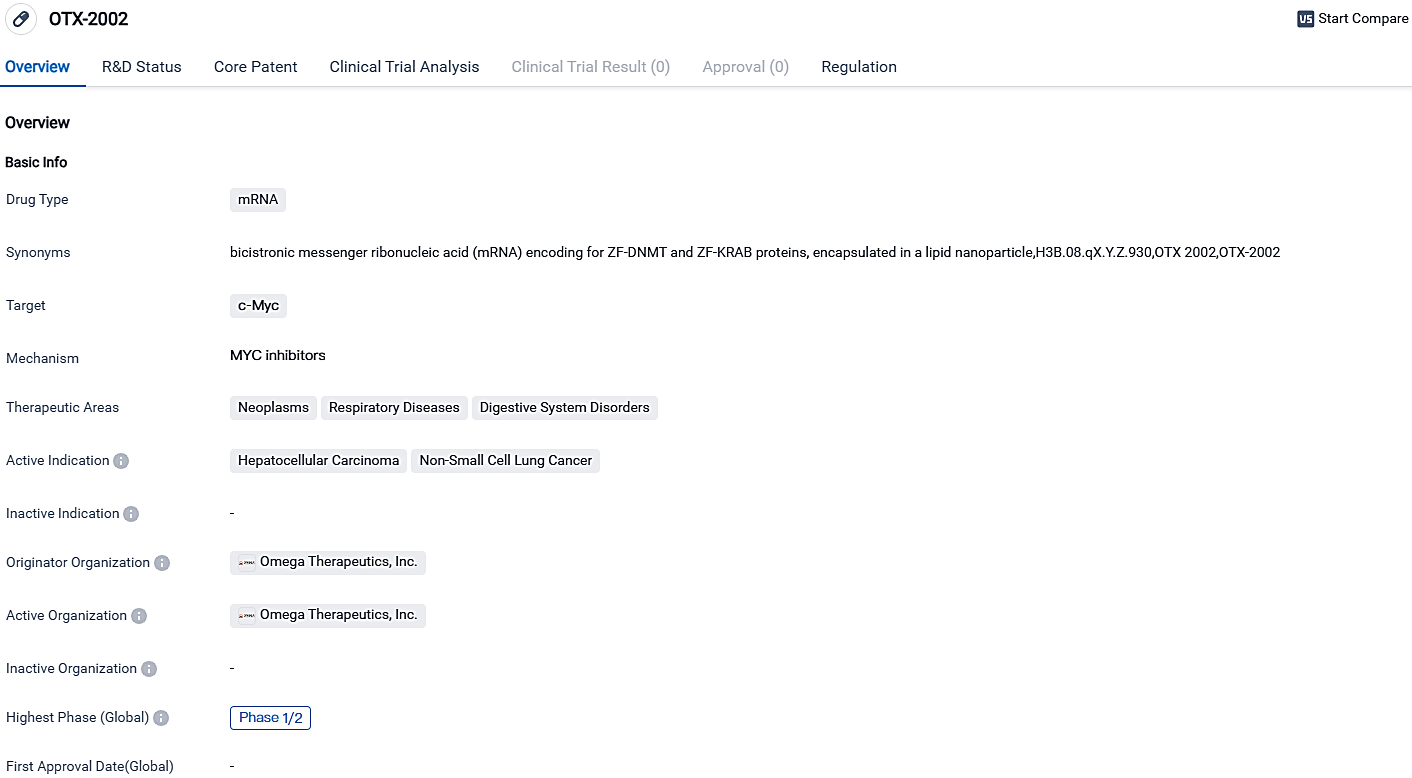
Omega Therapeutics Shares Optimistic Initial Clinical Results for OTX-2002 in the Continuing MYCHELANGELO™ I Trial
Omega Therapeutics, Inc., a front-runner in clinical-stage biotech, developing an innovative category of programmable epigenomic mRNA drugs, announced promising preliminary results concerning safety, tolerability, pharmacokinetic and translational data. These findings are based on the first two dose level groups from Part 1 of its continuing Phase 1/2 MYCHELANGELO™ I study. The study assesses OTX-2002 in patients suffering from hepatocellular carcinoma and various solid tumors linked with the c-MYC gene.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
OTX-2002, the primary developmental focus of the firm, is engineered to downregulate MYC pre-transcriptionally, a central oncogene involved in over half of all cancer types and about 70% of HCC incidents.
The encouraging preliminary results signify a key turning point for Omega, reinforcing the promise of our methodology and inaugurating a new epoch in therapeutic advancement using programmable mRNA candidates for guarded epigenomic manipulation,” commented Mahesh Karande, CEO of Omega Therapeutics.
Karande proceeded, "This is the inaugural occasion in a clinical environment where we've specifically zoned in on and judiciously adjusted the expression of the MYC oncogene, an oncology target that holds great promise but has proven challenging to efficiently treat by other means. We look forward to pushing forward with OTX-2002 as we strive to introduce a novel category of treatments for patients in need."
“All eight patients assessed at the preliminary low doses showed indisputable evidence of direct epigenetic alterations and corresponding swift, powerful, and lasting reductions in MYC mRNA expression levels,” contributed Thomas McCauley, Ph.D., Omega Therapeutics' Chief Scientific Officer. “Paired with positive safety data and stable pharmacokinetics, we're convinced that OTX-2002 could be potentially transformative for those suffering from HCC.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
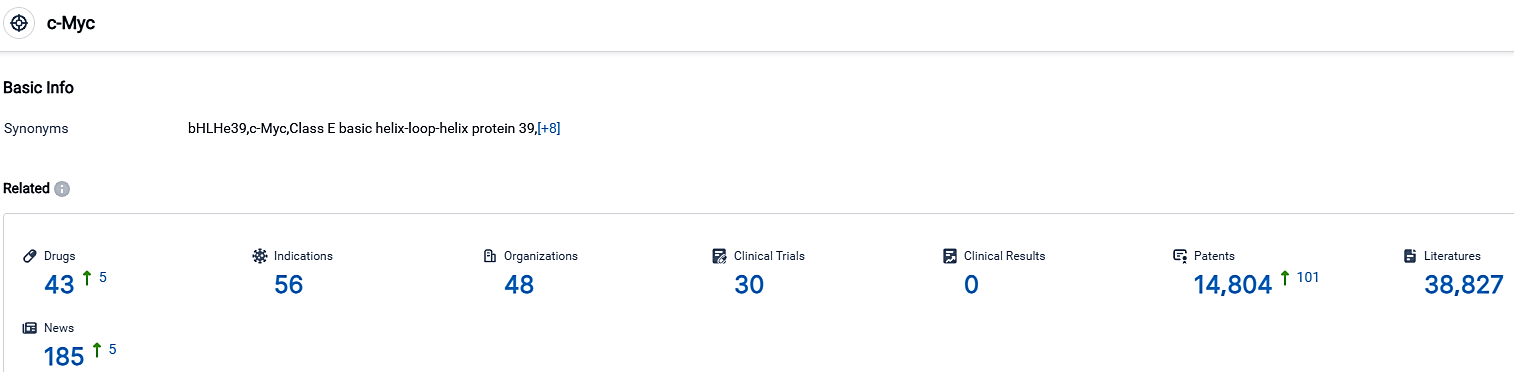
According to the data provided by the Synapse Database, As of October 1, 2023, there are 43 investigational drugs for the c-Myc target, including 56 indications,48 R&D institutions involved, with related clinical trials reaching 30,and as many as 14804 patents.
OTX-2002 is a therapeutic solution utilizing mRNA technology, conveyed through LNPs, with the aim to diminish MYC expression before the transcription process, by means of epigenetic modification, and may bypass MYC's self-regulation. It demonstrates significant potential as a prospective therapy for liver cancer known as hepatocellular carcinoma and non-small cell lung cancer. With its mRNA-based strategy and focused attack on the c-Myc protein, OTX-2002 stands out as a novel and pioneering pharmaceutical option.