Advances in Clinical Research on SOS1 inhibitors
SOS1, is a protein that plays a crucial role in signal transduction pathways within the human body. It acts as a guanine nucleotide exchange factor (GEF) for the Ras family of small GTPases, specifically Ras and Rap1. By catalyzing the exchange of GDP for GTP, SOS1 activates these GTPases, leading to the initiation of downstream signaling cascades involved in cell growth, differentiation, and survival. SOS1 is particularly important in the regulation of receptor tyrosine kinase signaling, making it a key player in various physiological processes and a potential target for therapeutic interventions in the pharmaceutical industry.
SOS1 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 25 Sep 2023, there are a total of 22 SOS1 drugs worldwide, from 27 organizations, covering 14 indications, and conducting 9 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.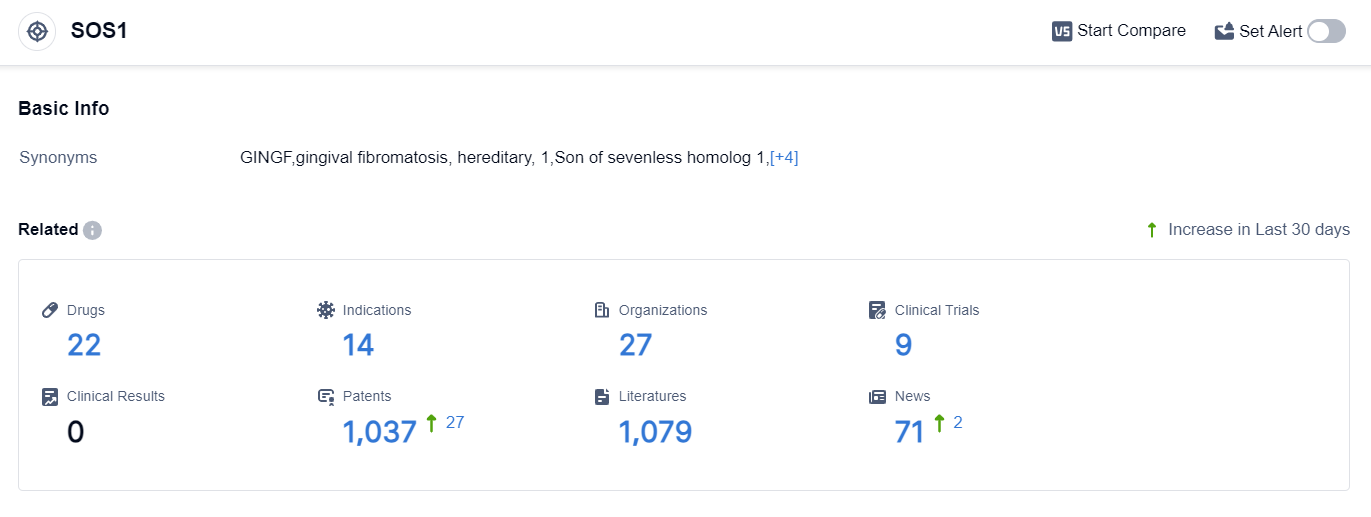
The analysis of the current competitive landscape of target SOS1 reveals that Suzhou Zelgen Biopharmaceuticals Co., Ltd., Mirati Therapeutics, Inc., C.H. Boehringer Sohn AG & Co. KG, and Revolution Medicines, Inc. are the leading companies in terms of R&D progress.
These companies are focusing on developing drugs for indications such as Colorectal Cancer, Non-Small Cell Lung Cancer, and Solid Tumors. Small molecule drugs are the most rapidly progressing drug type, with intense competition around the innovative drug.
China is showing significant progress in the development of drugs targeting SOS1, with a high number of drugs in Preclinical development. The future development of target SOS1 is promising, with ongoing research and development efforts by various companies and countries.
SOS1 inhibitors entering clinical phase I/II studies: MRTX-0902
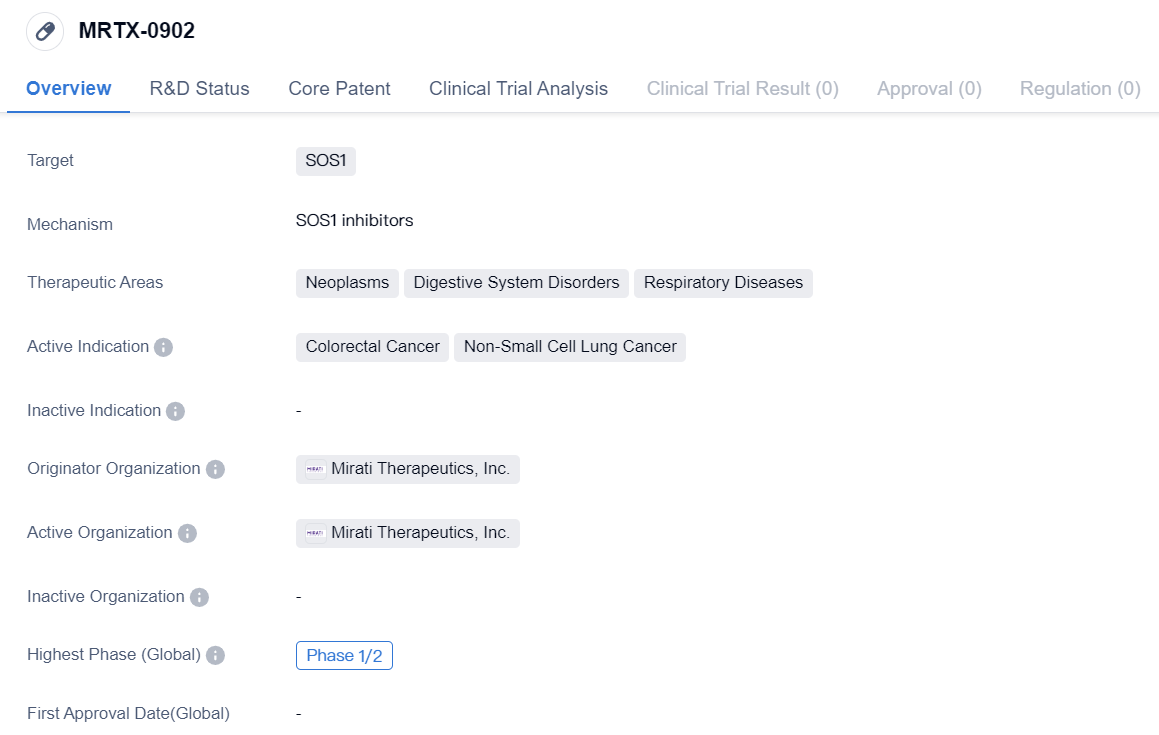
MRTX-0902 is a small molecule drug developed by Mirati Therapeutics, Inc. It falls under the category of biomedicine and is currently in Phase 1/2 of development. The drug specifically targets SOS1, a protein involved in cell signaling pathways.
The therapeutic areas that MRTX-0902 focuses on are neoplasms, digestive system disorders, and respiratory diseases. Neoplasms refer to abnormal growth of cells, commonly known as tumors. Digestive system disorders encompass a range of conditions affecting the gastrointestinal tract, such as inflammatory bowel disease or gastric ulcers. Respiratory diseases involve disorders of the respiratory system, including conditions like asthma or chronic obstructive pulmonary disease (COPD).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The active indications for MRTX-0902 are colorectal cancer and non-small cell lung cancer. Colorectal cancer is a type of cancer that affects the colon or rectum, while non-small cell lung cancer is a common form of lung cancer that accounts for the majority of cases.
Mirati Therapeutics, Inc. is the originator organization behind the development of MRTX-0902. As the originator, they are responsible for the initial discovery and development of the drug. Mirati Therapeutics, Inc. is a pharmaceutical company focused on developing targeted oncology therapies.
Overall, MRTX-0902 is a small molecule drug that targets SOS1 and is being developed by Mirati Therapeutics, Inc. It is currently in Phase 1/2 of development and shows potential for treating colorectal cancer and non-small cell lung cancer. The drug's therapeutic areas include neoplasms, digestive system disorders, and respiratory diseases.
ZG2001
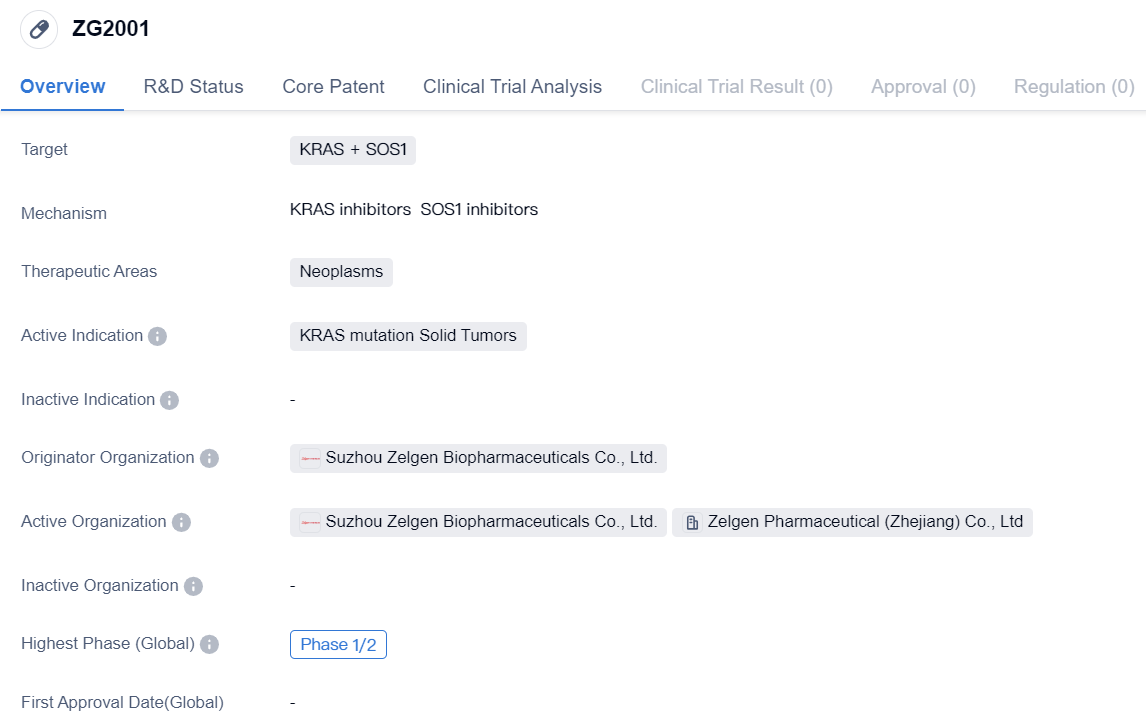
ZG2001 is a small molecule drug developed by Suzhou Zelgen Biopharmaceuticals Co., Ltd. It falls under the therapeutic area of neoplasms and specifically targets KRAS and SOS1. The drug is primarily indicated for the treatment of solid tumors with KRAS mutations.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Being a small molecule drug, ZG2001 is designed to interact with specific molecular targets within cells. In this case, it targets KRAS and SOS1, which are proteins involved in cell signaling pathways that regulate cell growth and division. By targeting these proteins, ZG2001 aims to inhibit the abnormal signaling caused by KRAS mutations, which are commonly found in various types of solid tumors.
Currently, ZG2001 is in Phase 1/2 of clinical development, both globally and in China. This indicates that the drug has undergone initial safety testing in humans and is now being evaluated for its efficacy and safety in a larger patient population. Phase 1/2 trials typically involve a relatively small number of patients and are conducted to determine the optimal dosage, assess the drug's effectiveness, and further evaluate its safety profile.
As an active indication, KRAS mutation solid tumors represent a significant unmet medical need. KRAS mutations are frequently observed in various types of cancers, including lung, colorectal, and pancreatic cancers. These mutations are associated with aggressive tumor growth, resistance to standard therapies, and poor patient outcomes. Therefore, the development of targeted therapies like ZG2001 holds great promise in improving the treatment options and outcomes for patients with KRAS mutation solid tumors.
In summary, ZG2001 is a small molecule drug developed by Suzhou Zelgen Biopharmaceuticals Co., Ltd. It targets KRAS and SOS1 and is indicated for the treatment of solid tumors with KRAS mutations. Currently in Phase 1/2 of clinical development, ZG2001 has the potential to address the significant unmet medical need in patients with KRAS mutation solid tumors.