Arcus Biosciences Reveals Partnership for Testing Casdatifan and Volrustomig Combo in Kidney Cancer Study
Arcus Biosciences, Inc. (NYSE:RCUS), a global biopharmaceutical company in the clinical development stage that specializes in creating unique molecules and combination therapies for cancer treatment, has announced a collaboration agreement for a clinical trial with AstraZeneca (LSE/STO/Nasdaq: AZN). This partnership will investigate the combined use of casdatifan (AB521), Arcus's investigational HIF-2a inhibitor, and volrustomig, AstraZeneca’s investigational PD-1/CTLA-4 bispecific antibody, in individuals diagnosed with ccRCC.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Terry Rosen, Ph.D., the CEO of Arcus, stated, “Based on the observed pharmacokinetic and pharmacodynamic profiles, together with emerging clinical data from our ARC-20 trial in ccRCC patients, we believe casdatifan holds best-in-class promise.” He added, “This partnership with AstraZeneca provides an opportunity to explore the potential of combining casdatifan with volrustomig to enhance patient outcomes in ccRCC.”
Cristian Massacesi, AstraZeneca’s Chief Medical and Oncology Development Officer, commented, “Renal cell carcinoma is known to respond to CTLA-4 inhibition, and our initial study using volrustomig alone showed promising efficacy in treating first-line advanced ccRCC.” He expressed enthusiasm about combining HIF-2a inhibition with volrustomig to achieve more profound and lasting responses for patients.
In this collaborative effort, AstraZeneca will take charge of sponsoring and conducting a study to examine the safety and preliminary efficacy of the casdatifan and volrustomig combination in advanced ccRCC patients.
This marks the second partnership between Arcus and AstraZeneca. Back in 2020, they announced a collaboration on PACIFIC-8, a Phase 3 registrational study involving domvanalimab-an Fc-silent anti-TIGIT antibody—combined with durvalumab, an anti-PD-L1 antibody, for patients with unresectable Stage III non-small cell lung cancer.
According to the agreement between Gilead and Arcus, Gilead retains the option to join the development and commercialization efforts for casdatifan following Arcus’s submission of a qualifying data package.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
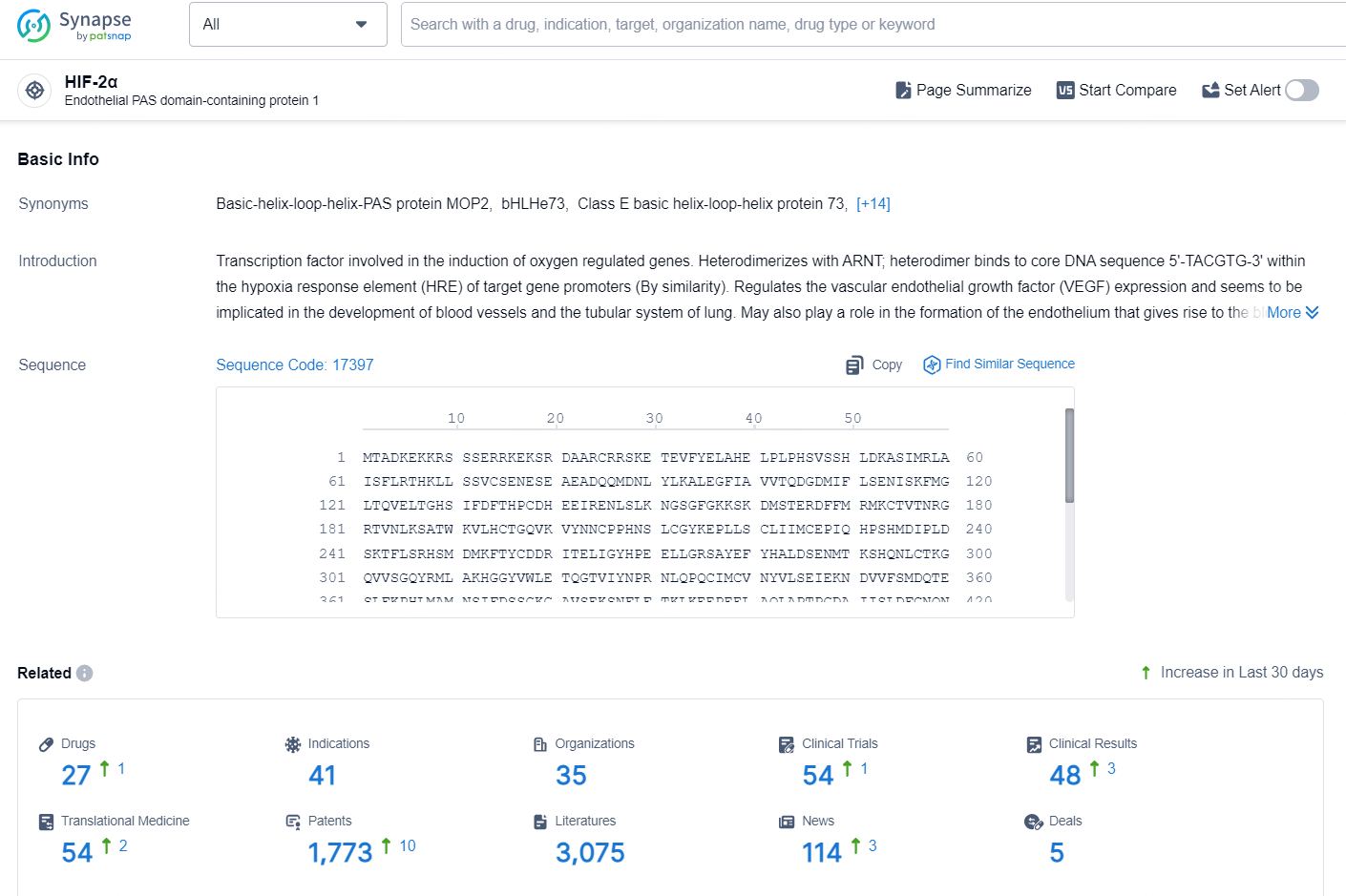
According to the data provided by the Synapse Chemical, As of October 9, 2024, there are 27 investigational drugs for the HIF-2α target, including 41 indications, 35 R&D institutions involved, with related clinical trials reaching 54, and as many as 1773 patents.
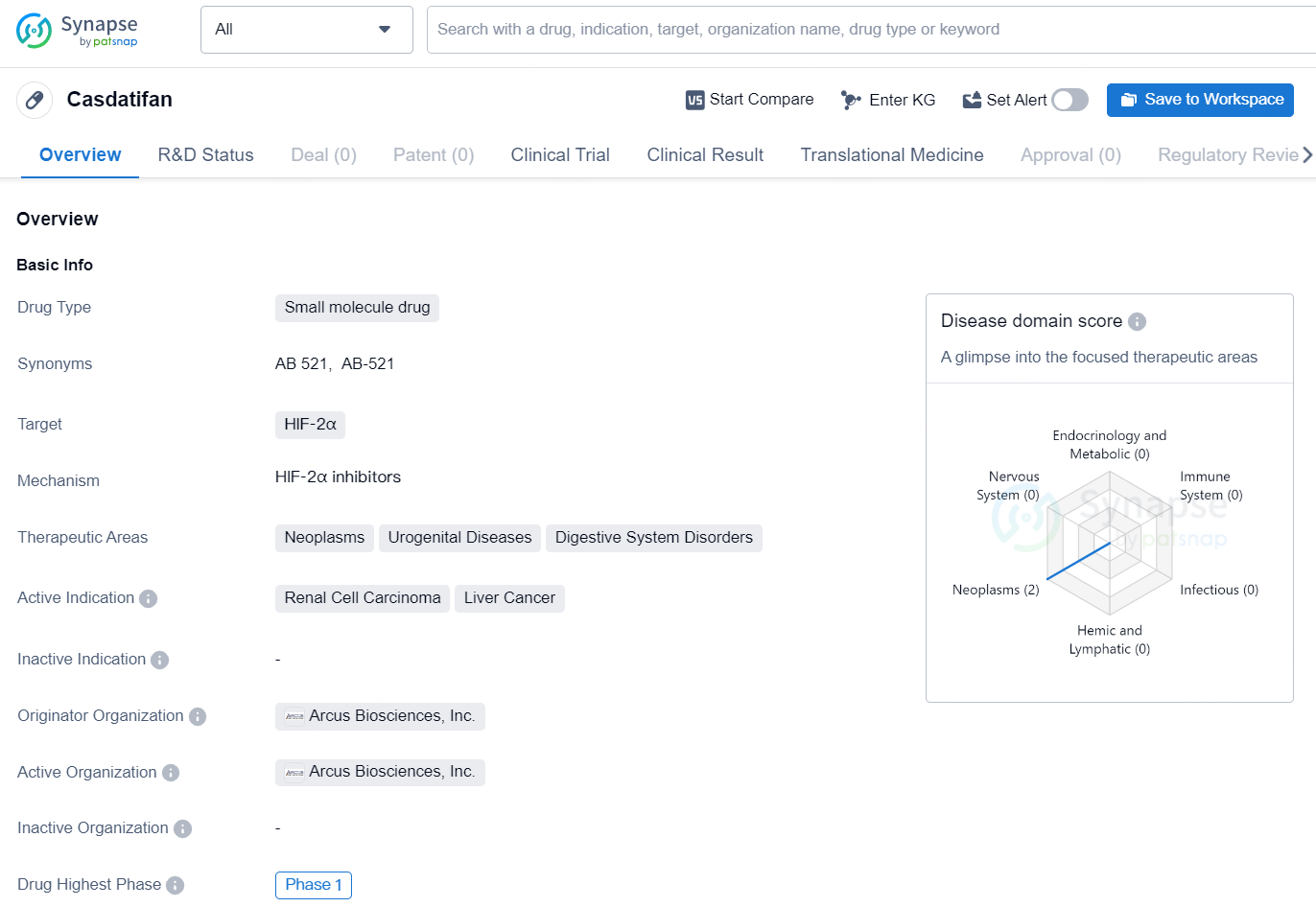
Casdatifan is a small molecule drug developed by Arcus Biosciences, Inc. It targets HIF-2α and falls within the therapeutic areas of neoplasms, urogenital diseases, and digestive system disorders. The active indications for Casdatifan include renal cell carcinoma and liver cancer. It has reached Phase 1 in its development, indicating that it has undergone initial testing in human subjects.