Dupixent becomes the first biologic drug approved in the U.S. for COPD treatment
The US Food and Drug Administration (FDA) has authorized the use of Dupixent (dupilumab) as an adjunct maintenance therapy for adults suffering from poorly managed chronic obstructive pulmonary disease (COPD) with an eosinophilic phenotype. Dupixent is the pioneering biologic drug sanctioned in the United States for the treatment of these patients.
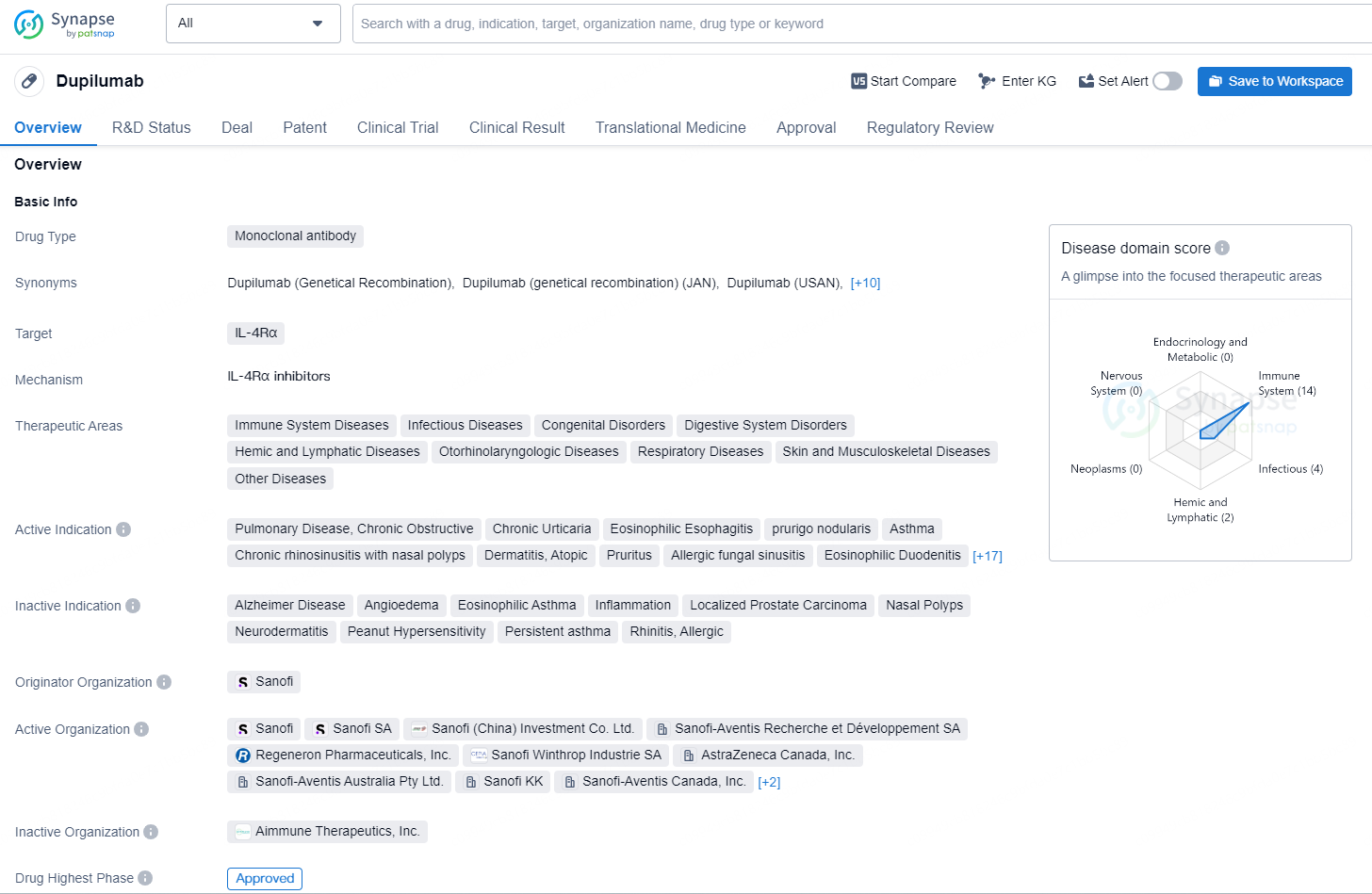
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Jean Wright, M.D.
Chief Executive Officer at The COPD Foundation
"Individuals with poorly managed COPD have been in need of new medications to alleviate their daily discomforts such as shortness of breath, coughing, wheezing, fatigue, and unexpected hospitalizations. These patients frequently face challenges with routine tasks that many people consider easy, like walking or running everyday errands. We are pleased with the approval of this new treatment to give patients an additional method to better control their condition."
Paul Hudson
Chief Executive Officer at Sanofi
“Dupixent has demonstrated its capacity to transform the treatment landscape of various diseases partially driven by type 2 inflammation with significant unmet medical needs, with one million patients worldwide being treated across currently approved indications. Today's approval marks another achievement for Dupixent, as it becomes the first and only approved add-on biologic therapy for inadequately controlled COPD, offering patients with this challenging disease the hope of improved breathing and fewer exacerbations.”
The safety outcomes in both studies largely aligned with the established safety profile of Dupixent in its approved uses. In the combined BOREAS and NOTUS study data, the most frequently reported adverse events (>2%) in patients using Dupixent compared to placebo included viral infection, headache, nasopharyngitis, back pain, diarrhea, arthralgia, urinary tract infection, reaction at the injection site, rhinitis, eosinophilia, toothache, and gastritis. Cholecystitis was noted less commonly, occurring in 0.6% of patients on Dupixent versus 0.1% of patients on placebo.
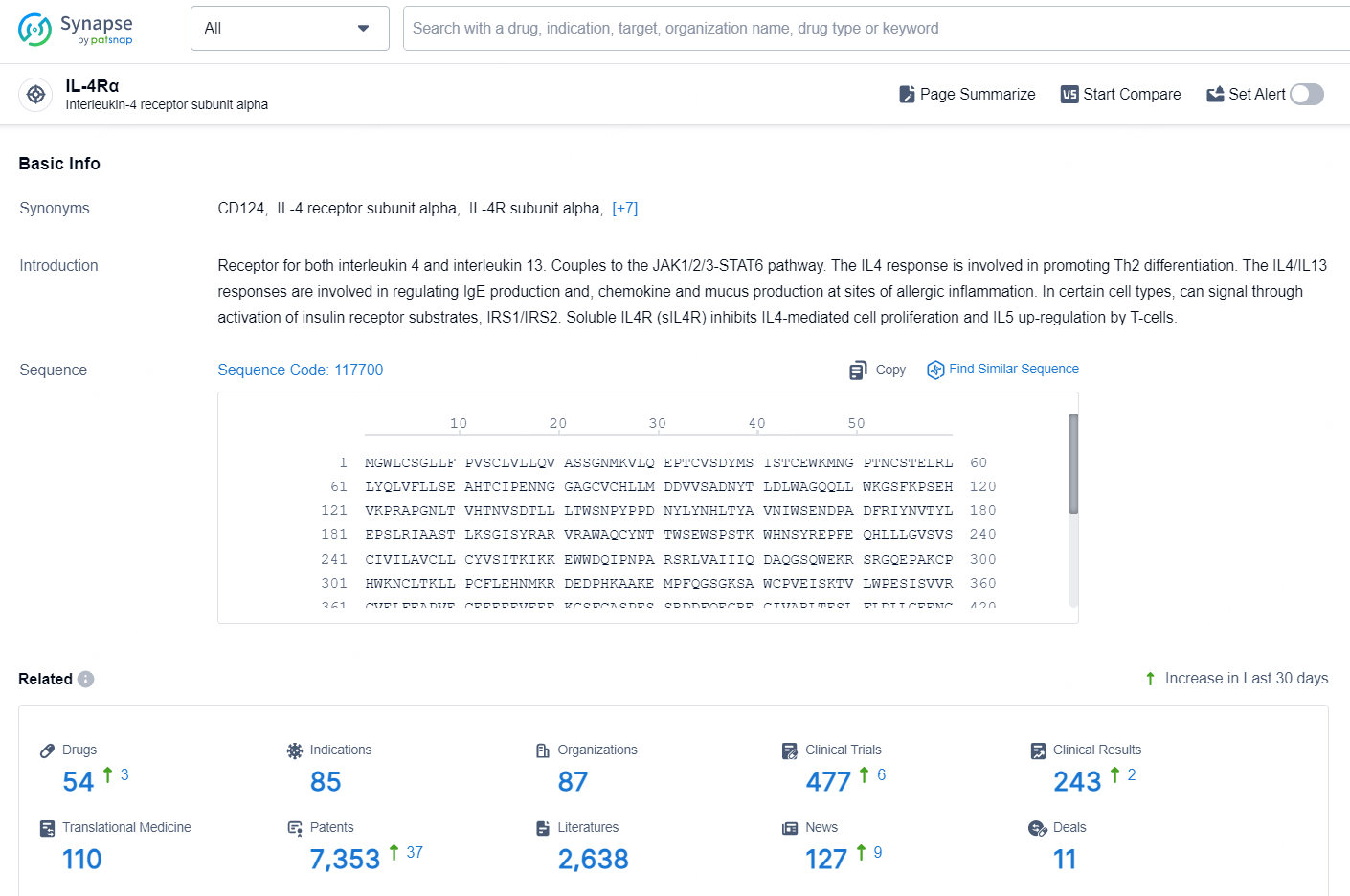
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 30, 2024, there are 54 investigational drugs for the IL-4Rα targets, including 85 indications, 87 R&D institutions involved, with related clinical trials reaching 477, and as many as 7353 patents.
Dupilumab is a monoclonal antibody drug targeting IL-4Rα with a wide range of therapeutic indications across various disease areas. It has been approved in both the global and Chinese markets, with its first approval in the United States in 2017, and is regulated under priority review and breakthrough therapy designations, among others.