FDA Approves Bristol Myers Squibb’s COBENFY™ for Adult Schizophrenia Treatment
Bristol Myers Squibb (NYSE: BMY) revealed that the U.S. Food and Drug Administration (FDA) has sanctioned COBENFY™ (xanomeline and trospium chloride), an oral drug designed for adult schizophrenia treatment. COBENFY marks the introduction of a novel class of medication after many decades and pioneers a unique method for treating schizophrenia by specifically targeting M1 and M4 receptors in the brain, while not inhibiting D2 receptors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Chris Boerner, PhD, chairman of the board and CEO at Bristol Myers Squibb, remarked, "Receiving approval for our innovative treatment for schizophrenia is a significant achievement, introducing a groundbreaking pharmacological method that could transform the current treatment model for the condition after over three decades." He added, "As we make our return to neuropsychiatry, our commitment is to reshape the discourse surrounding severe mental illnesses, starting with this new approval for schizophrenia."
Schizophrenia is a long-lasting and frequently disabling mental disorder that influences a person's thoughts, emotions, and behaviors. Approximately 2.8 million individuals in the United States are affected by this condition. Symptoms often manifest in early adulthood and vary widely among individuals, making diagnosis and management challenging. Although current treatment standards can help control schizophrenia symptoms, up to 60% of patients experience either insufficient symptom relief or unmanageable side effects.
Gordon Lavigne, CEO of the Schizophrenia & Psychosis Action Alliance, said, "For those living with schizophrenia, finding an effective treatment can be challenging. Offering a range of treatment options empowers patients and healthcare providers to better manage this serious disease." He continued, "Individuals with schizophrenia deserve and seek more from their treatments. Today's approval delivers a new alternative, aiding those with schizophrenia as they receive proper support to rebuild their lives."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
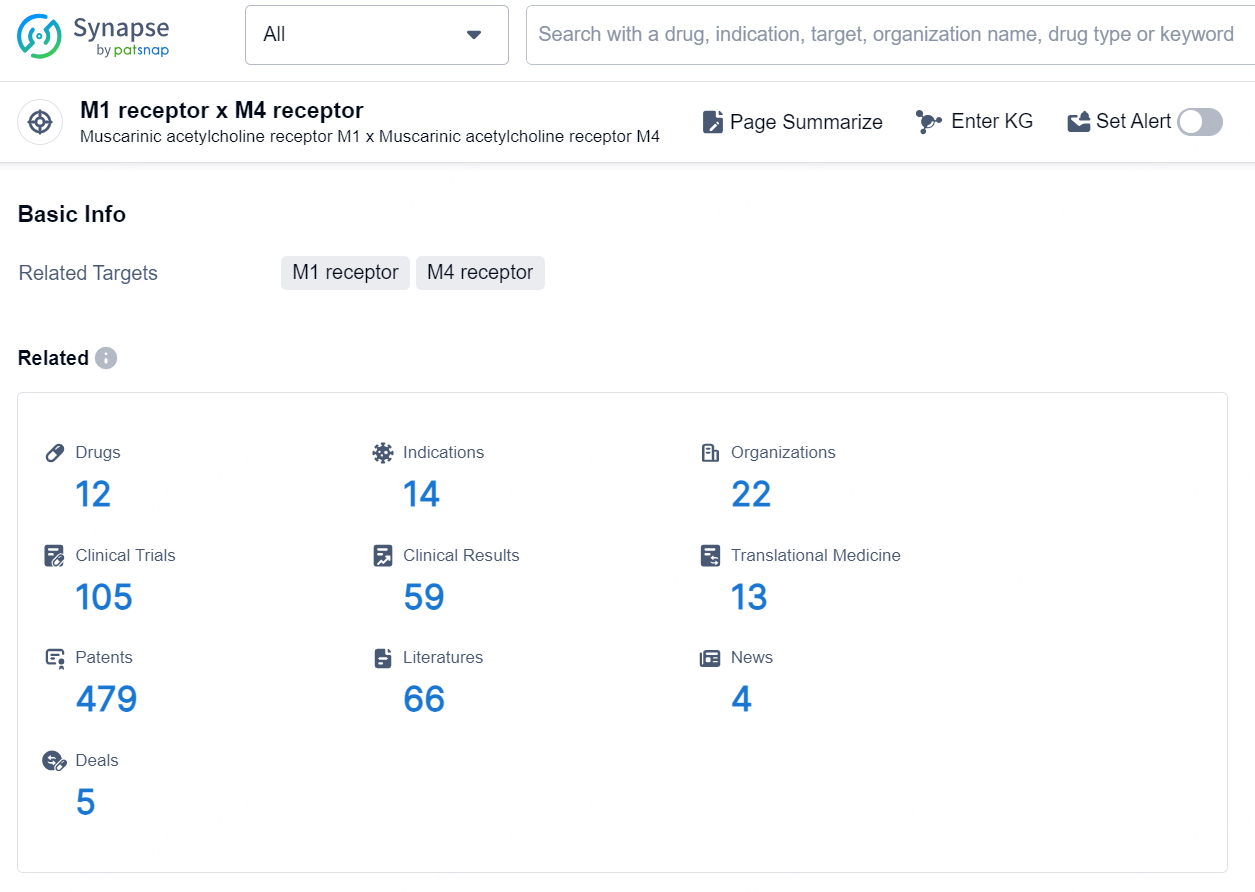
According to the data provided by the Synapse Database, As of September 30, 2024, there are 12 investigational drugs for the M1 and M4 receptor targets, including 14 indications, 22 R&D institutions involved, with related clinical trials reaching 105, and as many as 479 patents.
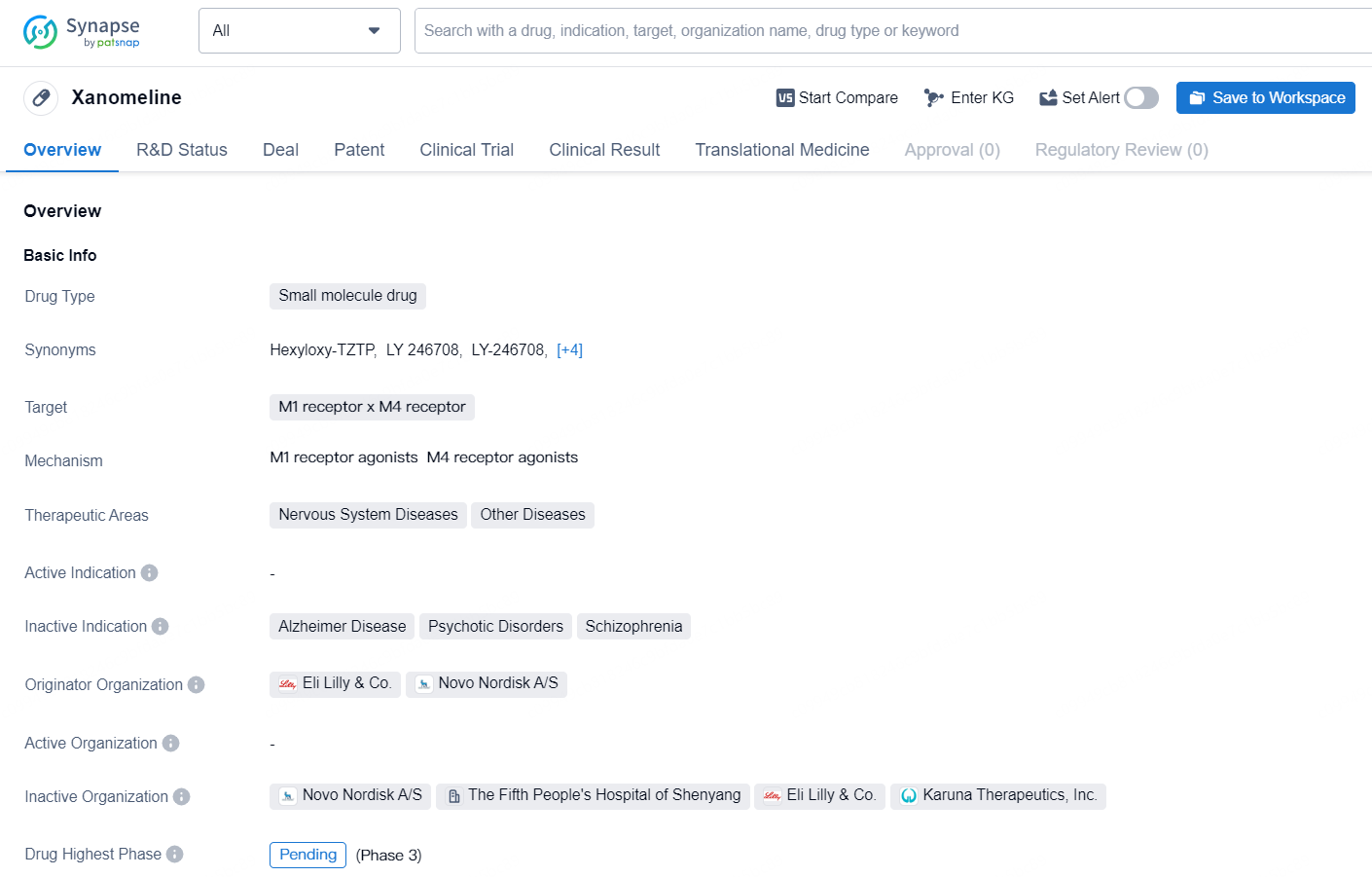
Xanomeline is a small molecule drug that targets the M1 and M4 receptors and falls within the therapeutic areas of Nervous System Diseases and Other Diseases. The drug is currently in a pending phase, representing the highest phase of development on a global scale. Xanomeline was developed by Eli Lilly & Co. and Novo Nordisk A/S.