Arrowhead Pharmaceuticals Begins Phase 1/2a Trial of ARO-INHBE to Treat Obesity
Arrowhead Pharmaceuticals, Inc. (NASDAQ: ARWR) has announced that it has administered doses to the first participants in a Phase 1/2a clinical trial for ARO-INHBE, an investigational RNA interference (RNAi) therapy that is being explored as a potential obesity treatment. Additionally, Arrowhead has submitted a request for regulatory approval to start a clinical trial for its second candidate targeting obesity, ARO-ALK7. Both ARO-INHBE and ARO-ALK7 are aimed at disrupting a recognized pathway that instructs the body to store fat in adipose tissue.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
ARO-INHBE represents a significant initiative for Arrowhead, aligning with our strategic commitment to the development and commercialization of vital RNAi-based therapies aimed at treating cardiometabolic disorders. "Our preclinical investigations have demonstrated encouraging outcomes for this innovative mechanism that aims to decrease body weight while possibly preserving lean muscle mass, leading to an enhancement in body composition," stated James Hamilton, M.D., Chief of Discovery and Translational Medicine at Arrowhead. "The Phase 1/2 trial will assess ARO-INHBE as a standalone treatment in part 1 and in combination with tirzepatide in part 2, with participation from patients diagnosed with obesity in both segments."
The design of ARO-INHBE involves the reduction of hepatic INHBE gene expression along with its secreted product, Activin E. This gene, INHBE, is a promising target validated through genetic studies; in humans, loss-of-function variants of INHBE correlate with better fat distribution and a decreased chance of metabolic diseases, including type 2 diabetes. Activin E serves as a ligand within a pathway that governs energy balance in adipose tissue. By inhibiting this pathway through the experimental treatment ARO-INHBE, there is potential to enhance lipolysis and diminish issues related to adipose hypertrophy and dysfunction, as well as visceral fat and insulin resistance.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
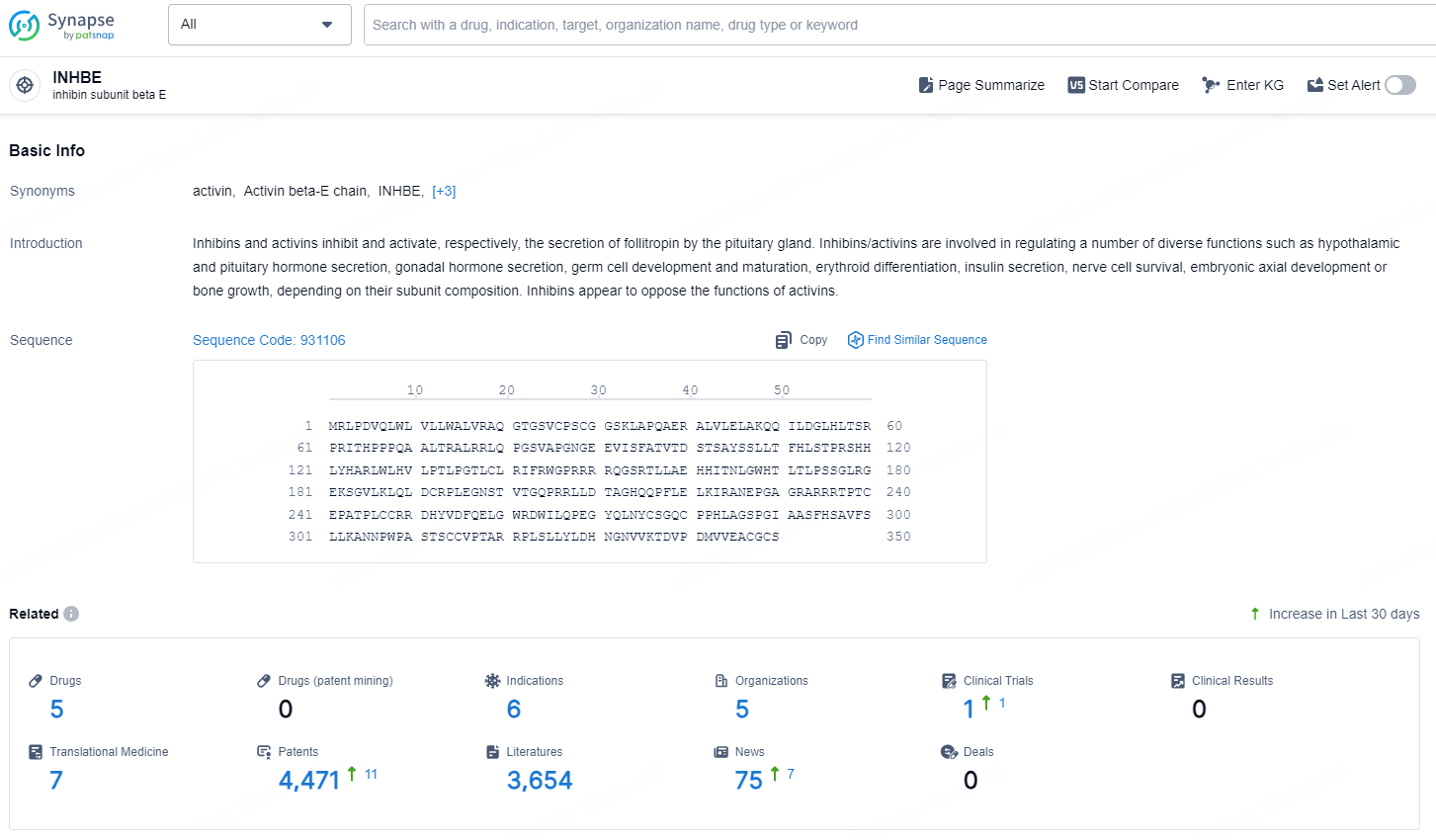
According to the data provided by the Synapse Chemical, As of December 30, 2024, there are 5 investigational drugs for the INHBE target, including 6 indications, 5 R&D institutions involved, with related clinical trial reaching 1, and as many as 4471 patents.
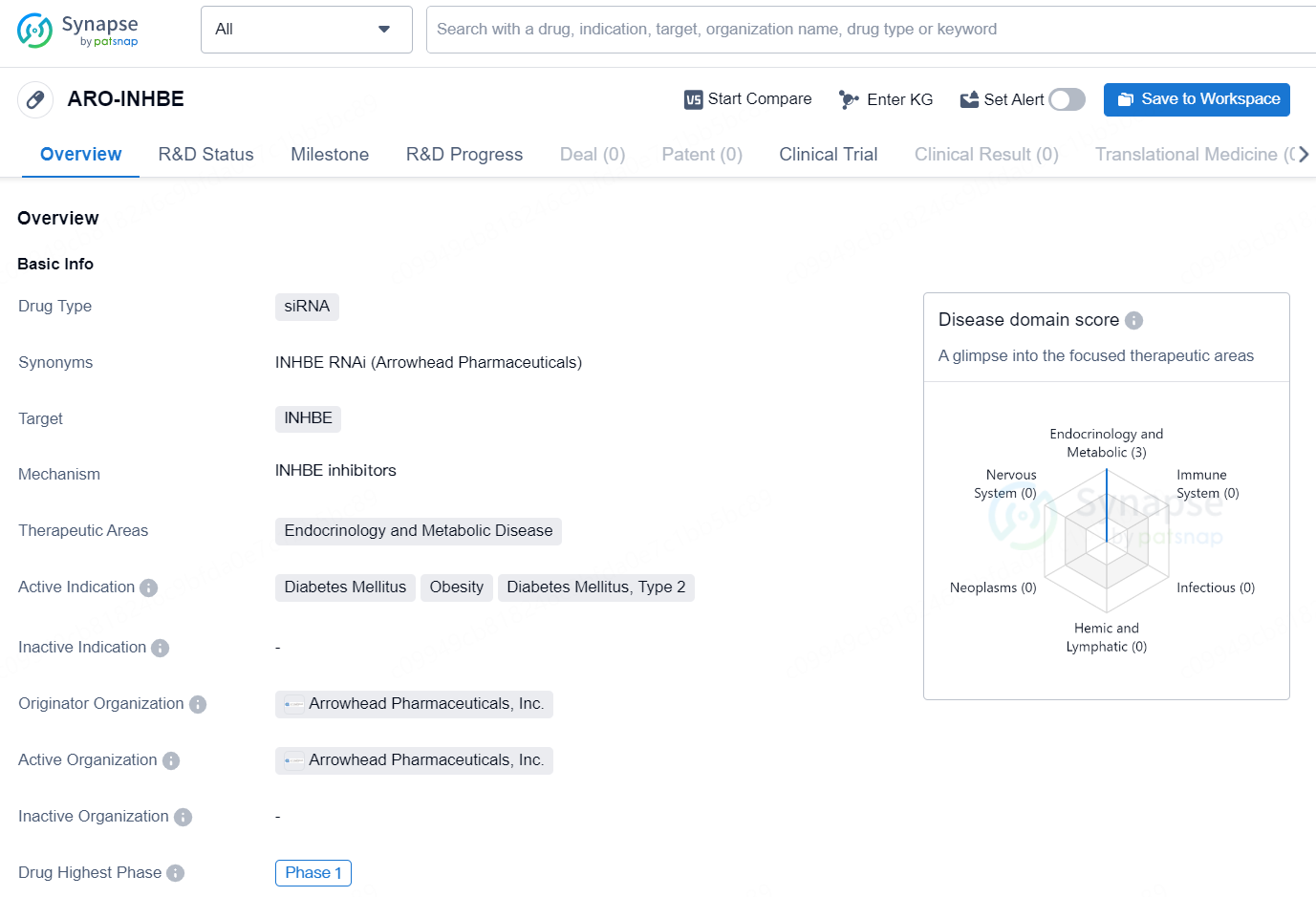
The drug ARO-INHBE is a siRNA type drug developed by Arrowhead Pharmaceuticals, Inc. The drug targets INHBE and is intended for use in the therapeutic areas of endocrinology and metabolic disease. The active indications for this drug include diabetes mellitus, obesity, and type 2 diabetes. The highest phase globally for ARO-INHBE is Phase 1.