ARTHEx Biotech Begins Phase I-IIa Trial Dosing with ArthemiR™ for Myotonic Dystrophy Type 1
ARTHEx Biotech S.L., a biotechnology firm in the clinical phase dedicated to creating innovative therapies via gene expression modulation, has announced that the initial participant has received a dose in the Randomized Placebo Controlled Double Blind Phase I-IIa ArthemiR™ Trial of ATX-01 targeting Myotonic Dystrophy Type 1 (DM1). DM1 is a severe rare neuromuscular condition that leads to muscle weakness and various life-threatening complications. Presently, there are no approved treatments that modify the disease for DM1.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
ATX-01 marks the first therapeutic approach in the industry aimed at targeting anti-microRNA for the treatment of DM1, specifically focusing on microRNA 23b (miR-23b). This microRNA is known to regulate the expression of muscle blind-like (MBNL) proteins, which are crucial for proper mRNA splicing and consequently for the synthesis of various proteins within the body. The pathology of DM1 arises primarily from the toxic accumulation of dystrophia myotonica protein kinase (DMPK) mRNA in the nucleus, sequestering the MBNL proteins. Additionally, the overexpression of miR-23b leads to the translational repression of MBNL, resulting in a decreased availability of MBNL to perform its regulatory functions. ATX-01 is distinct as it employs a dual mechanism of action: (1) it promotes the production of MBNL, and (2) it destabilizes the toxic DMPK aggregates, which decreases DMPK mRNA levels and releases the sequestered MBNL. Together, these effects contribute to an overall increase in active MBNL levels, facilitating splicing corrections.
"Since ARTHEx's inception, we have dedicated ourselves to finding a novel therapeutic solution for individuals with DM1 and their families. We are thrilled to mark a significant milestone in our development with the initiation of dosing for the first participant in our ArthemiR™ trial," stated Dr. Frédéric Legros, Chairman and CEO of ARTHEx. "The opportunity in the marketplace for a new DM1 therapy is compelling, given the substantial unmet medical needs and the absence of an approved disease-modifying option."
"ATX-01 is an exceptional molecule — entirely discovered and developed by ARTHEx's team through extensive research and development efforts. We are eager to explore the potential of its dual action mechanism to enhance the quality of life for DM1 patients," remarked Dr. Beatriz Llamusí, Chief Scientific Officer and Co-Founder of ARTHEx.
The ArthemiR™ trial is a global Phase I-IIa study that is double-blind, placebo-controlled, and assesses both single and multiple ascending doses in individuals with classic DM1. The main aim is to evaluate the safety and tolerability of ATX-01 in this patient population. ARTHEx will also assess target engagement in muscle tissue via biomarkers, such as MBNL levels and RNA splicing indices. Clinical endpoints will involve metrics on muscle functionality, patient-reported outcomes, and quality of life assessments. The study anticipates enrolling fifty-six participants to facilitate the evaluation of ATX-01's efficacy in this cohort.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
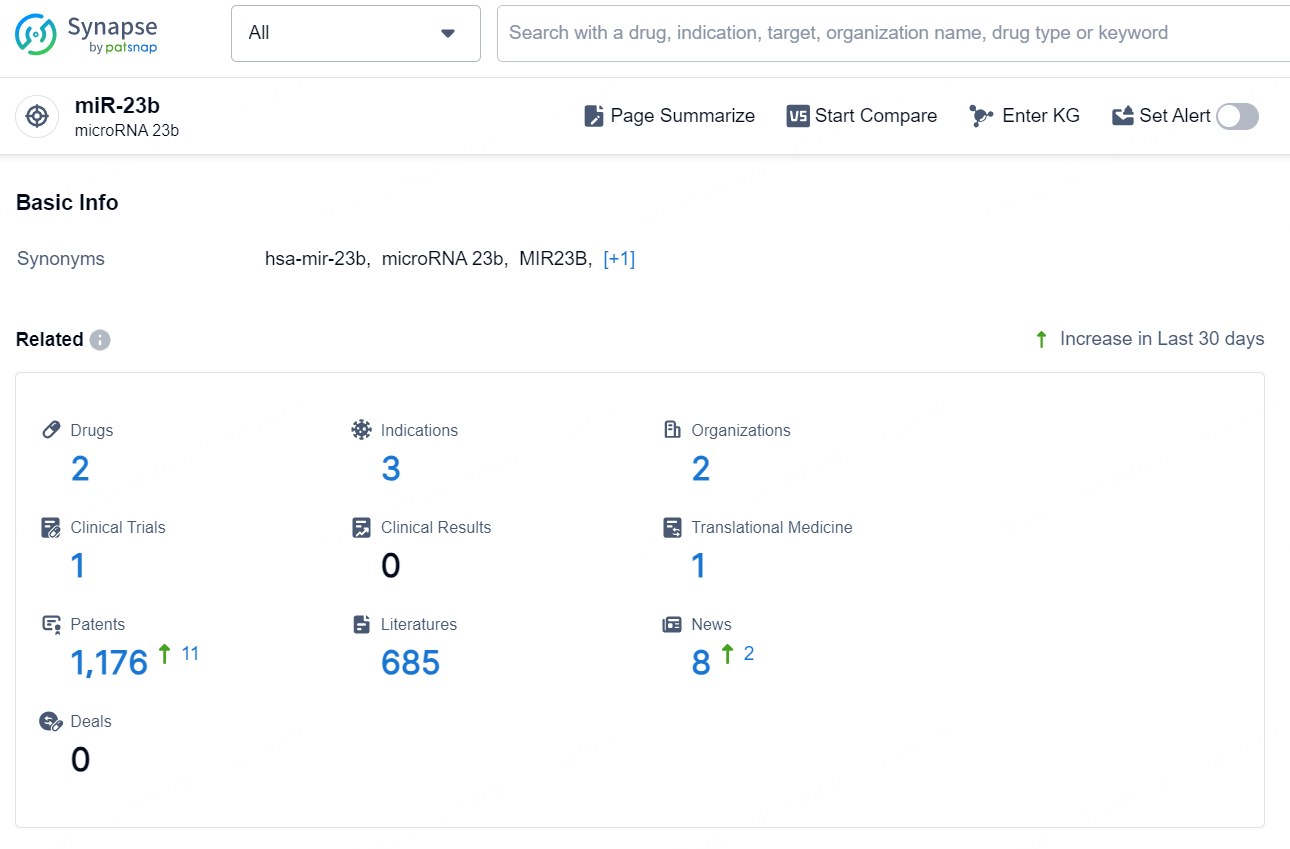
According to the data provided by the Synapse Chemical, As of October 23, 2024, there are 2 investigational drugs for the miR-23b target, including 3 indications, 2 R&D institutions involved, with related clinical trial reaching 1, and as many as 1176 patents.
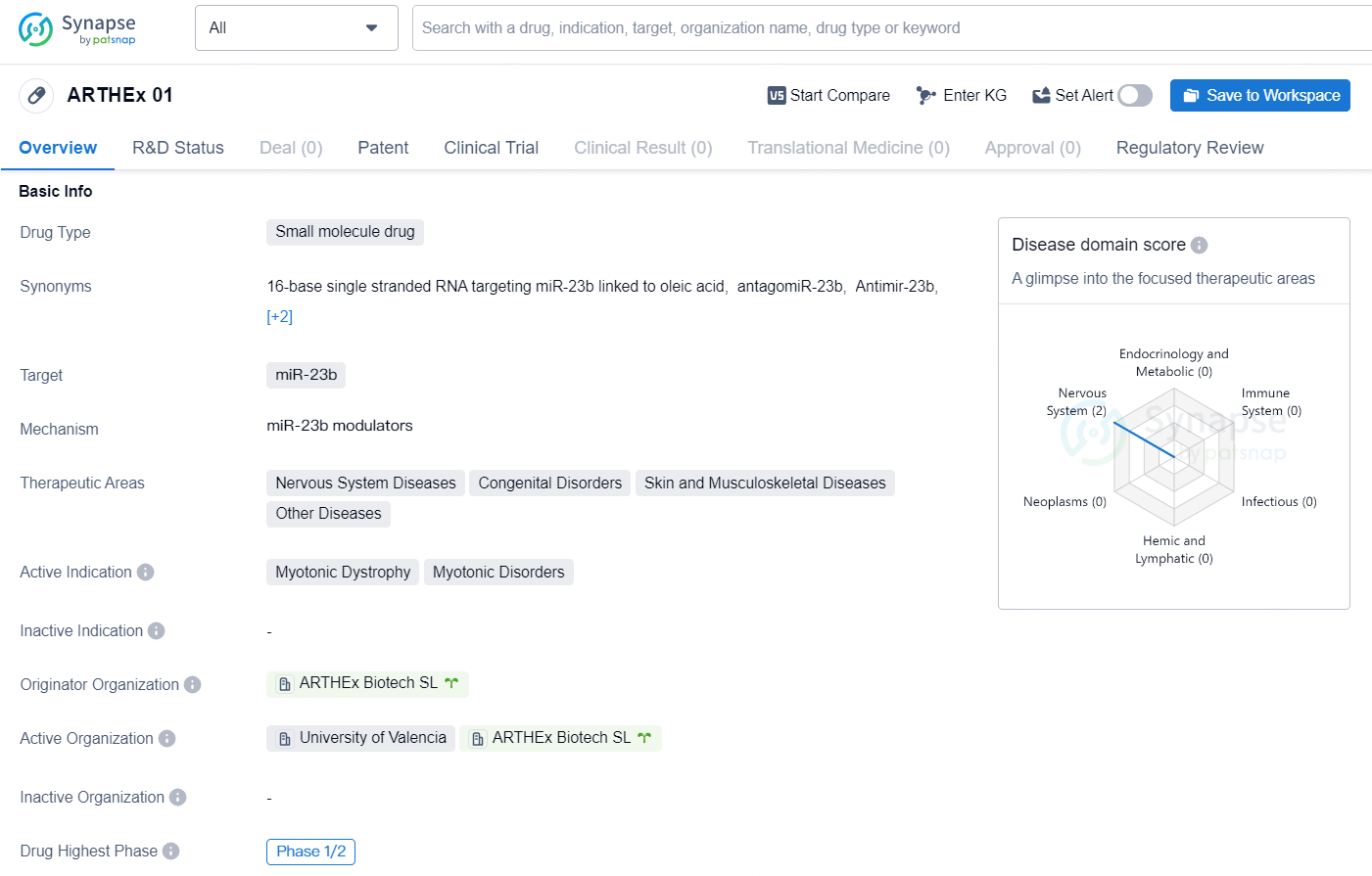
The drug ARTHEx 01 is a small molecule drug designed to target miR-23b, with a focus on addressing Nervous System Diseases, Congenital Disorders, Skin and Musculoskeletal Diseases, and Other Diseases. Its active indication is Myotonic Dystrophy and Myotonic Disorders. The drug is being developed by ARTHEx Biotech SL and is currently in Phase 1/2 of clinical trials, indicating that it has shown promise in early-stage testing.