BioAegis Therapeutics Begins Phase 2 Trial of Gelsolin for ARDS Treatment
BioAegis Therapeutics, an innovative biotechnology firm specializing in advanced treatments for inflammatory conditions, is excited to share that the initial participant has been enrolled in the Phase 2 clinical trial of rhu-pGSN aimed at addressing Acute Respiratory Distress Syndrome (ARDS) (NCT05947955).
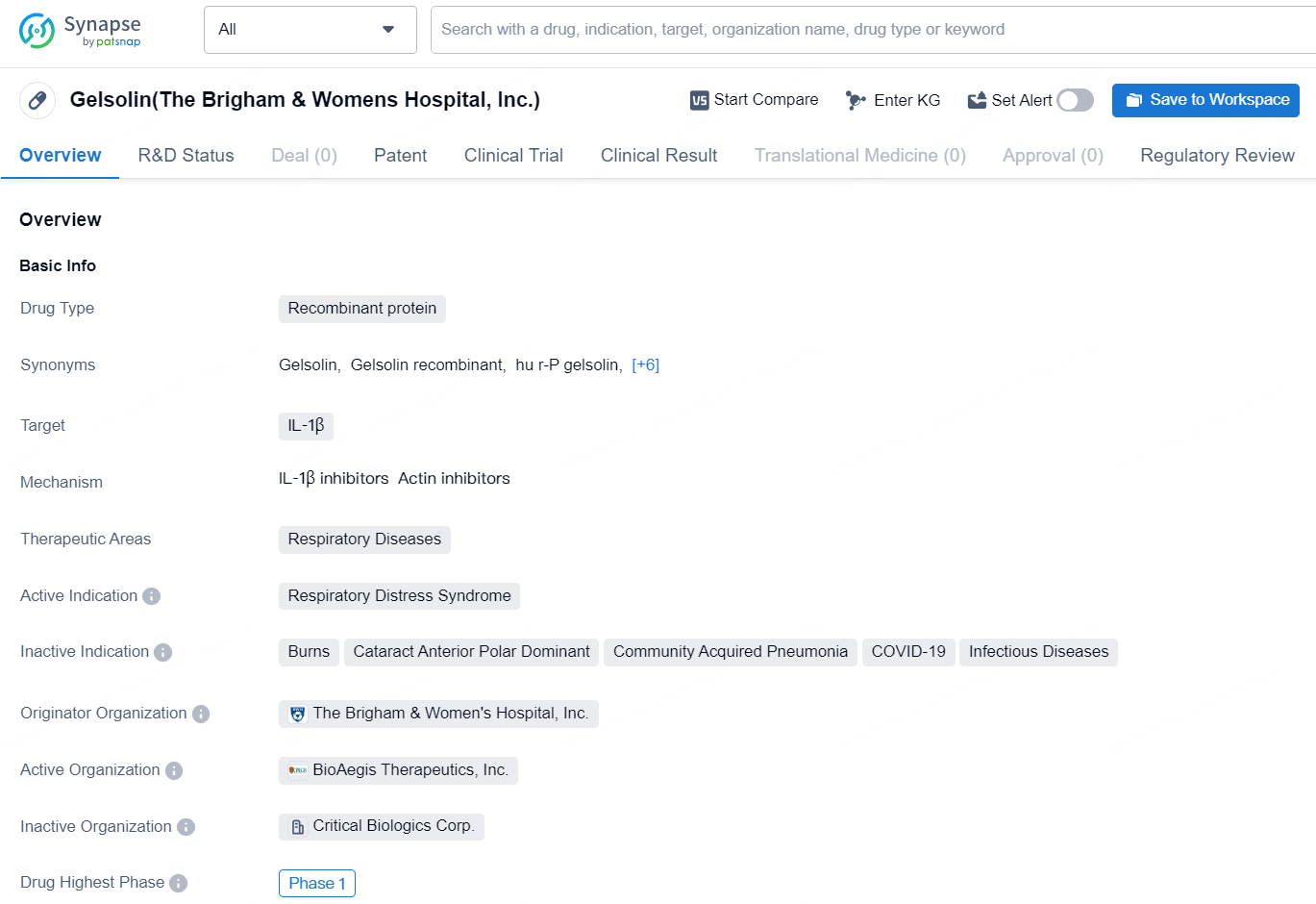
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Phase 2 Clinical Trial of rhu-pGSN
This randomized, double-blind, placebo-controlled proof-of-concept study aims to assess the effectiveness of rhu-pGSN, along with standard care, by focusing on the rate of survival without organ failure at Day 28. The trial involves administering six intravenous doses of rhu-pGSN to hospitalized patients experiencing moderate to severe ARDS (P/F ratio ≤150) as a result of infection. Additionally, the study will evaluate the safety, tolerability, and various secondary endpoints related to the treatment.
A total of seventy-five research sites across the United States, Canada, the United Kingdom, and European countries such as Belgium, France, Italy, Germany, and the Netherlands have been chosen for this investigation. The goal is to enroll 600 participants.
This initiative has received partial funding from the federal government, specifically from the U.S. Department of Health and Human Services, the Administration for Strategic Preparedness and Response, and the Biomedical Advanced Research and Development Authority (BARDA), under contract number 75A50123C00067.
Effectively Addressing ARDS to Mitigate Society's Susceptibility to Global Pandemics
Acute Respiratory Distress Syndrome (ARDS) can emerge as a serious complication arising from sepsis, trauma, pneumonia, or other infectious diseases, leading to critical lung damage with fluid accumulation. This condition hampers respiratory function, necessitating supplemental oxygen, mechanical ventilation, and comprehensive critical care, thus imposing a considerable strain on healthcare systems.
Despite robust medical interventions, a significant portion of ARDS patients do not survive, and survivors often face lasting complications, such as decreased lung capacity and a lower quality of life. In the United States, ARDS impacts over 500,000 individuals annually, representing around 10% of all ICU admissions. The mortality rate associated with ARDS stands at roughly 40%. The absence of effective treatment options, combined with the condition's associated high mortality rate due to excessive inflammation, highlights an urgent need for novel therapeutic solutions in this area.
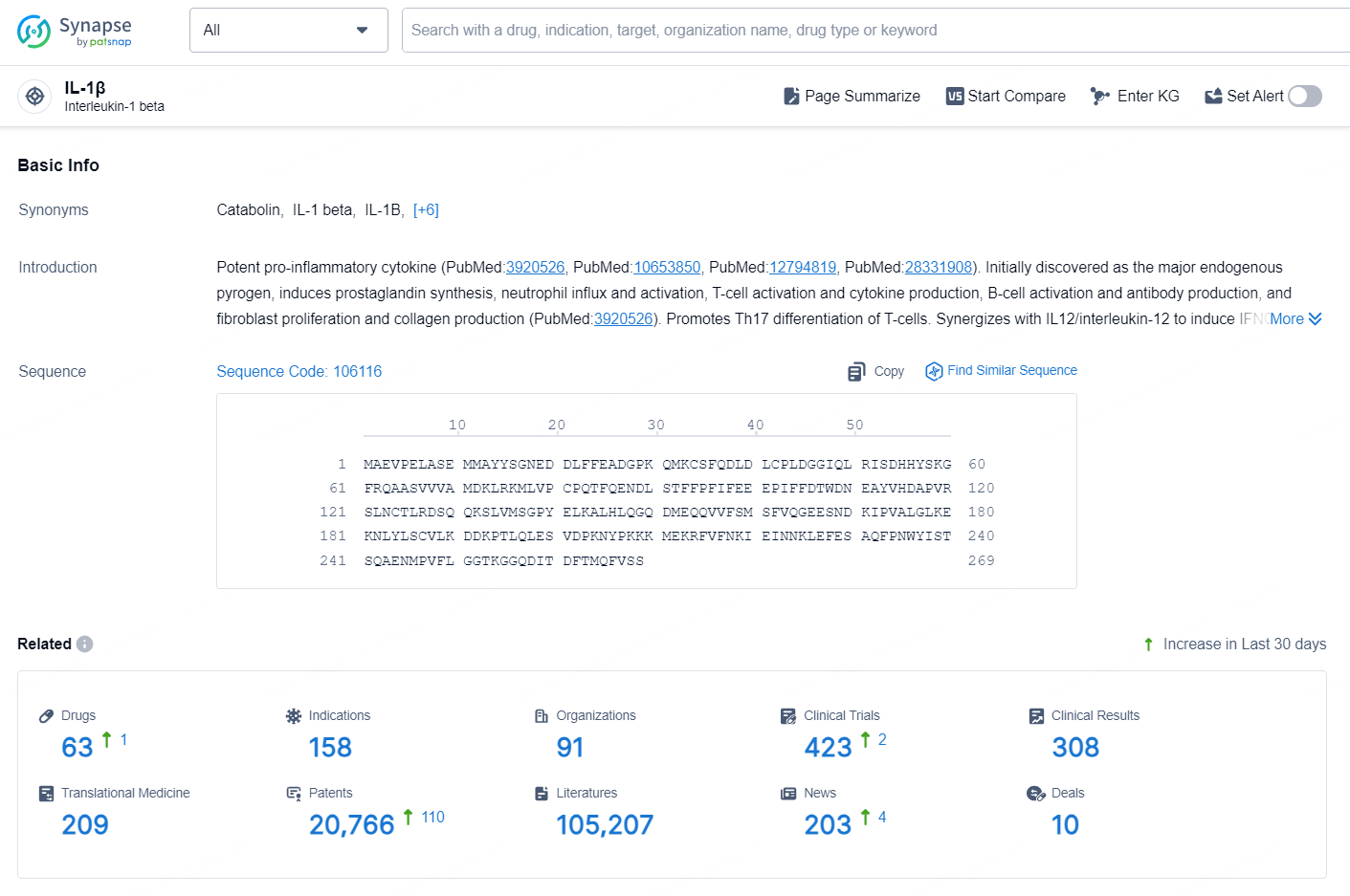
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 21, 2024, there are 63 investigational drugs for the IL-1β targets, including 158 indications, 91 R&D institutions involved, with related clinical trials reaching 423, and as many as 20766 patents.
The drug Gelsolin, developed by The Brigham & Women's Hospital, Inc., is a recombinant protein that targets IL-1β. It is being developed for the treatment of respiratory diseases, with a specific focus on Respiratory Distress Syndrome. The highest phase of development for Gelsolin is Phase 1, indicating that it is still in the early stages of clinical testing.