FDA Approves VYALEV™ for Advanced Parkinson’s in Adults
AbbVie (NYSE: ABBV) has announced that the U.S. Food and Drug Administration (FDA) has granted approval for VYALEV™ (foscarbidopa and foslevodopa). This marks it as the first and sole subcutaneous continuous 24-hour infusion therapy based on levodopa for managing motor fluctuations in adults suffering from advanced Parkinson’s disease (PD).
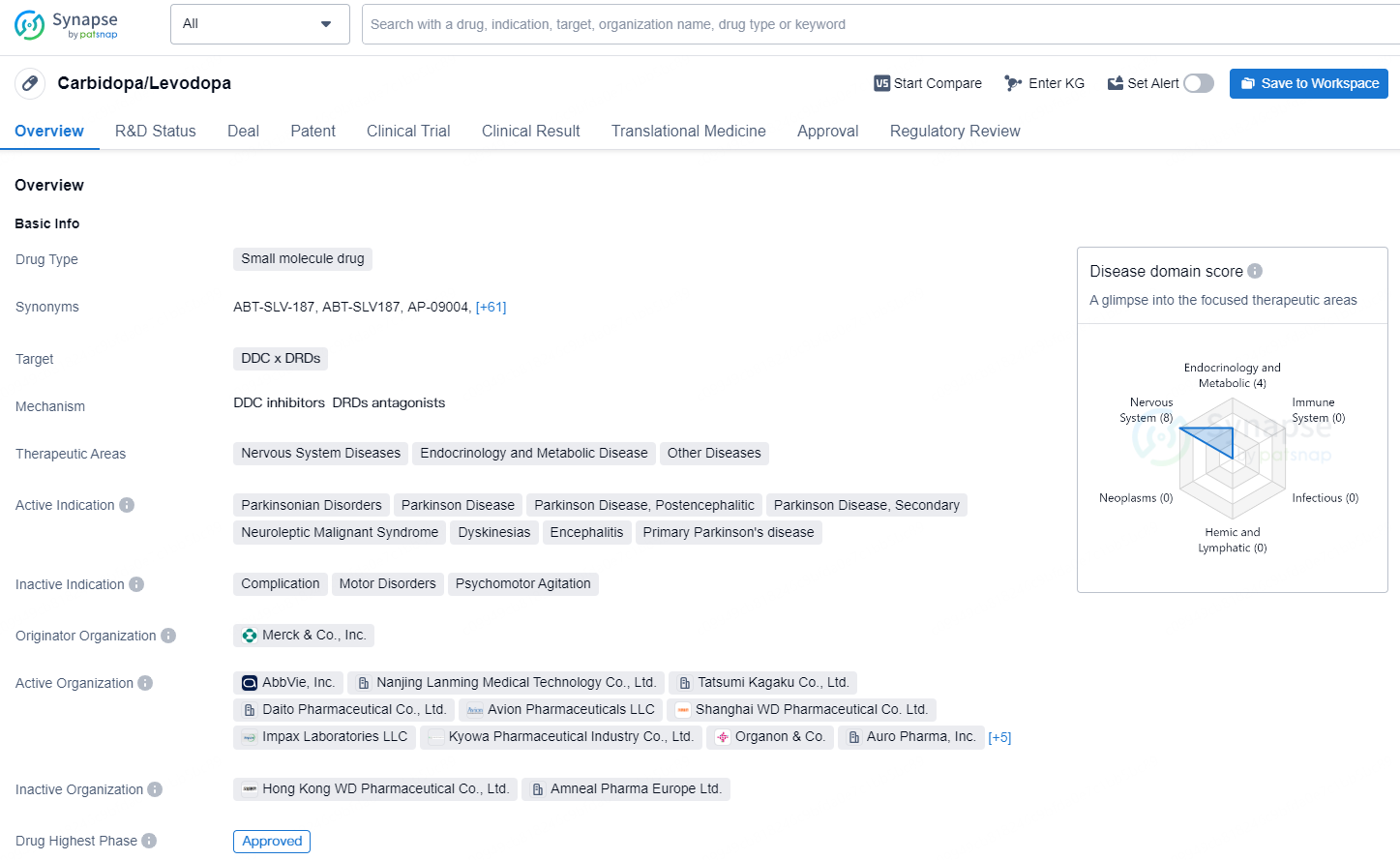
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"For an extended period, individuals affected by Parkinson's disease have faced a scarcity of treatment alternatives for advanced stages of the condition. As the disease progresses, oral medications often become less effective at managing motor symptoms, necessitating potential surgical interventions," stated Robert A. Hauser, M.D., MBA, a Neurology professor and Director of the Parkinson’s and Movement Disorder Center at the University of South Florida. "This innovative, non-invasive approach offers continuous levodopa delivery around the clock."
The approval of this treatment was based on a pivotal Phase 3 study lasting 12 weeks, which assessed the effectiveness of continuous subcutaneous infusion of VYALEV in adults with advanced Parkinson's disease, in comparison to oral immediate-release carbidopa/levodopa (CD/LD IR).1 Additionally, a 52-week open-label trial evaluated the long-term safety and effectiveness of VYALEV.
Results from the initial study indicated that patients treated with VYALEV experienced greater improvements in motor fluctuations, featuring increased "on" time without problematic dyskinesia and reduced "off" time, when compared to those on oral CD/LD IR.1 "On" time represents the periods of effective motor symptom management, whereas "off" time occurs when symptoms re-emerge.
Most of the adverse reactions (ARs) associated with VYALEV were classified as mild or moderate and non-serious. The most common adverse effects (occurring in 10 percent or more of patients and exceeding the incidence seen with CD/LD IR) included infusion site issues, hallucinations, and dyskinesia.
"Individuals with advanced Parkinson’s disease face daily hurdles due to the unpredictability in managing motor fluctuations, particularly as their condition advances," remarked Roopal Thakkar, M.D., executive vice president for research & development and chief scientific officer at AbbVie. "We are excited to offer this advancement to patients who may benefit from the continuous 24-hour delivery of VYALEV for better motor symptom control."
Parkinson’s disease is a chronic, progressive movement disorder characterized by tremors, muscle stiffness, slowed movements, and balance difficulties, stemming from the degeneration of dopamine-producing cells in the brain.
The timeline for patients to access VYALEV will vary based on their individual insurance plans, with coverage for Medicare beneficiaries anticipated in the latter half of 2025.
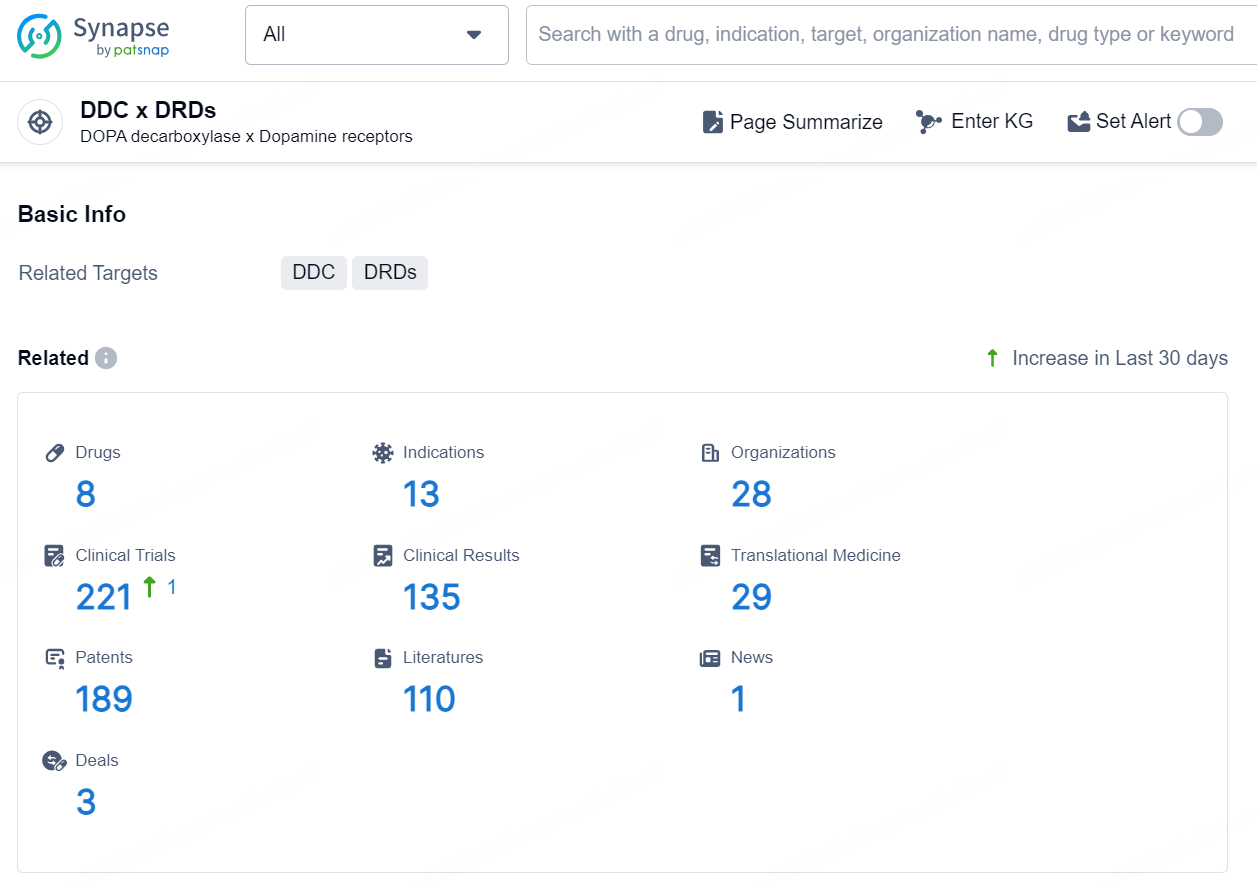
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 21, 2024, there are 35 investigational drugs for the DDC x DRDs targets, including 26 indications, 50 R&D institutions involved, with related clinical trials reaching 137, and as many as 9179 patents.
Carbidopa/Levodopa is a small molecule drug that targets the DDC x DRDs and is used in the therapeutic areas of Nervous System Diseases, Endocrinology and Metabolic Disease, as well as Other Diseases. It is indicated for the treatment of Parkinsonian Disorders, Parkinson Disease, Parkinson Disease Postencephalitic, Parkinson Disease Secondary, Neuroleptic Malignant Syndrome, Dyskinesias, Encephalitis, and Primary Parkinson's disease.