Poseida Therapeutics reveals a new CAR-T candidate as part of its partnership with Roche
Poseida Therapeutics, Inc. (Nasdaq: PSTX), a company engaged in clinical-stage allogeneic cell therapy and genetic medicines, focuses on developing unique non-viral therapies for individuals suffering from cancer, autoimmune disorders, and rare diseases. The firm revealed the introduction of a new development candidate as part of its partnership with Roche. This nomination has led to the activation of a $15 million milestone payment from Roche to Poseida.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
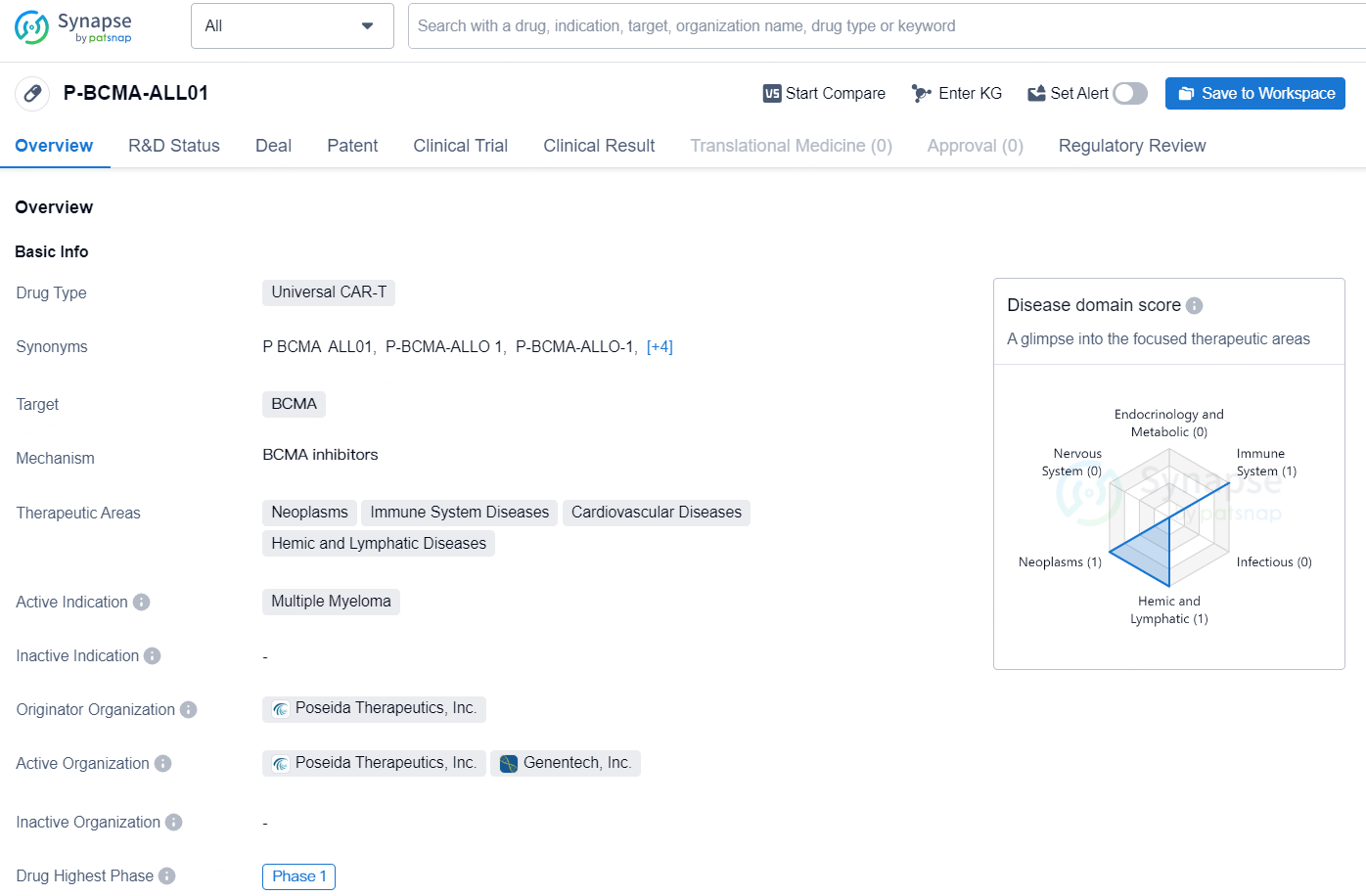
The newly identified candidate represents an allogeneic, dual CAR-T treatment designed to target specific antigens that are prevalent in hematologic cancers, such as multiple myeloma. The robust capabilities of Poseida's non-viral transposon-based DNA delivery mechanism allow for the introduction of genes that code for two complete chimeric antigen receptors (CARs) into T stem cell memory cells (TSCM).
"The selection of a new development candidate is a continuation of our partnership with Roche, showcasing the distinctive potential of our proprietary non-viral genetic engineering tools for producing diverse, TSCM-rich allogeneic CAR-T therapies aimed at one or several antigens," stated Kristin Yarema, Ph.D., president and CEO of Poseida Therapeutics. "Multiple myeloma is a prevalent and untreatable blood cancer that presents a significant opportunity for effective, safe, and accessible new treatments to broaden usage across different treatment lines and care settings. With strong preclinical evidence supporting this dual CAR-T target combination, we are eager to move this initiative forward into clinical stages as part of our collaboration. We also anticipate sharing updates regarding our CAR-T programs and earlier-stage developments during Poseida's upcoming Cell Therapy R&D Day."
Currently, Poseida and Roche are collaborating on three programs, initiated in August 2022, aimed at developing allogeneic CAR-T therapies for hematologic malignancies. The primary collaborative program, P-BCMA-ALLO1, targets BCMA and has been granted Regenerative Medicine Advanced Therapy (RMAT) designation for adult individuals with relapsed or refractory multiple myeloma after three or more treatment lines. Poseida is actively recruiting patients for the Phase 1b segment of the clinical trial. The second program, P-CD19CD20-ALLO1, is an allogeneic dual CAR-T candidate that is under Phase 1 development for B-cell cancers.
Dr. Yarema further remarked, "Given the encouraging differentiated safety and efficacy outcomes of P-BCMA-ALLO1 demonstrated in recent interim Phase 1 data presented at the IMS Annual Meeting in September, and in association with Roche, we are eager to proceed with patient enrollment in the Phase 1b trial. The newly introduced allogeneic dual CAR-T therapy candidate will utilize the same proprietary Poseida GMP manufacturing platform that was employed in the development and advancement of P-BCMA-ALLO1."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
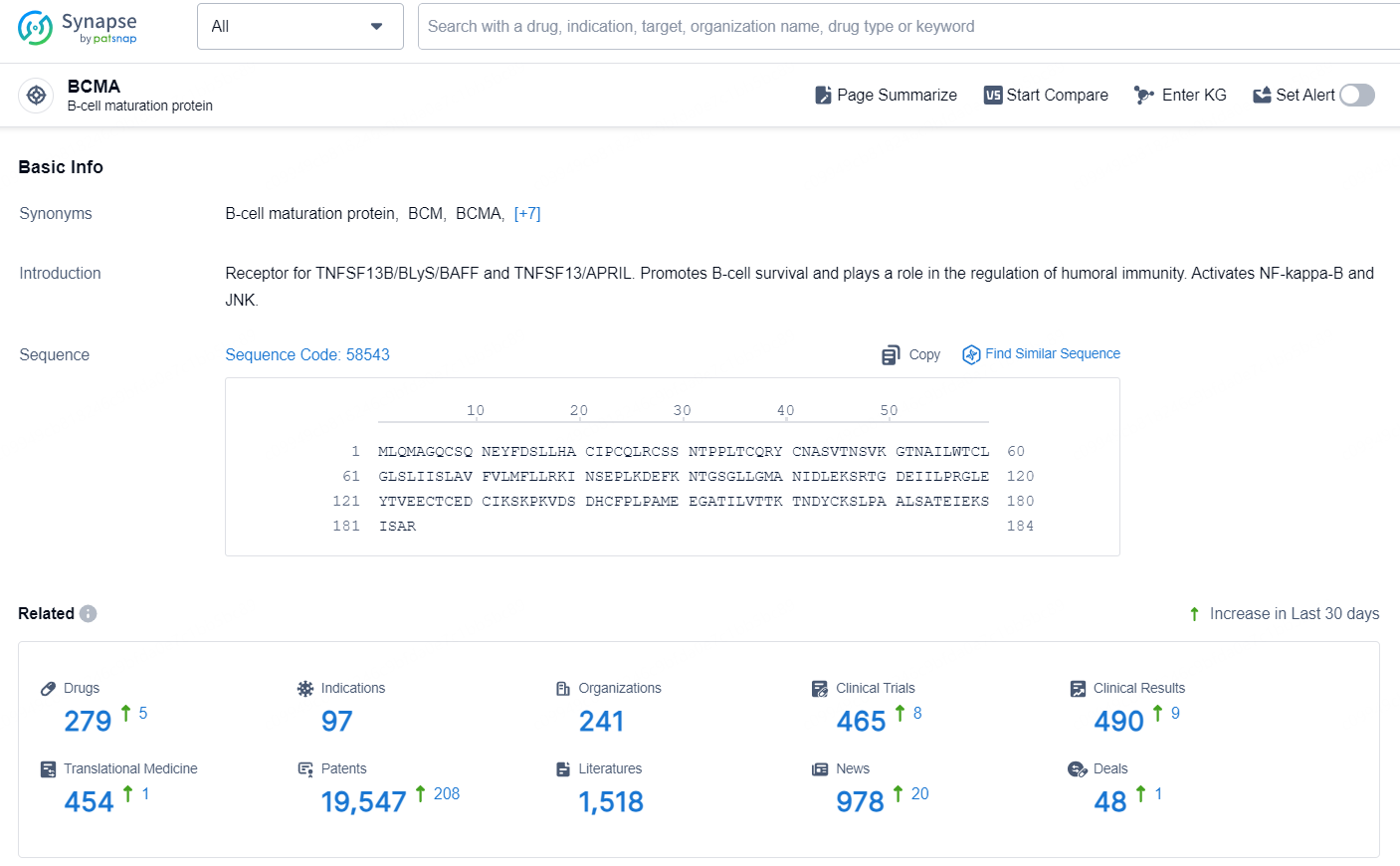
According to the data provided by the Synapse Database, As of October 21, 2024, there are 279 investigational drugs for the BCMA targets, including 97 indications, 241 R&D institutions involved, with related clinical trials reaching 465, and as many as 19547 patents.
The drug P-BCMA-ALL01 is a Universal CAR-T therapy that targets BCMA. It is being developed by Poseida Therapeutics, Inc. and is currently in Phase 1 of clinical trials. The drug is intended for the treatment of Multiple Myeloma, a type of neoplasm affecting the immune system, cardiovascular, and hemic and lymphatic systems.