Arvinas' two AR receptor PROTAC therapies show positive results in Phase 1/2 clinical trials
Recently, Arvinas Inc. announced positive efficacy and tolerance results of its PROTAC protein degrader targeting androgen receptor (AR), bavdegalutamide (ARV-110), and its second-generation AR-targeted PROTAC degrader ARV-766 in the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC) in Phase 1/2 clinical trials at the European Society for Medical Oncology (ESMO). Based on this data, Arvinas plans to prioritize the initiation of Phase 3 clinical trials for ARV-766 in the treatment of mCRPC and is scheduled to have related discussions with regulatory bodies in the second quarter of 2024.
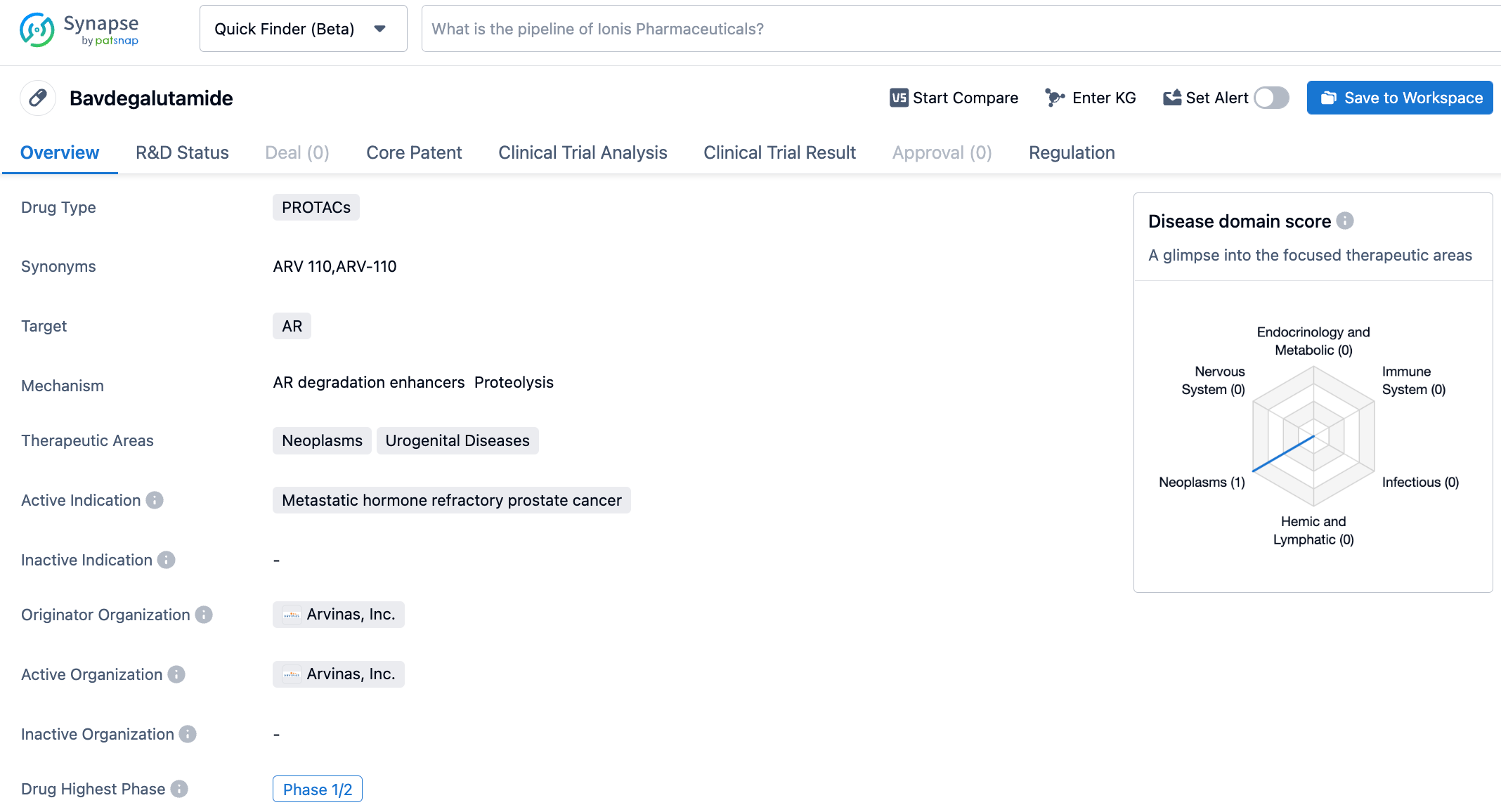
ARV-110 is a PROTAC drug developed by Arvinas for the treatment of prostate cancer. It demonstrated satisfactory safety and tolerance in Phase I clinical trials. ARV-110 is the first PROTAC to enter clinical trials. Its oral administration can effectively degrade wild-type AR and AR variants induced by antiandrogen treatment. In various prostate cancer cell lines, ARV-110 can degrade 95-98% of ARs. In drug-resistant models, ARV-110 can reduce tumor growth by 70-100%. Preliminary data from the Phase 1 clinical trials indicate that ARV-110 displays satisfactory safety and tolerance in patients.
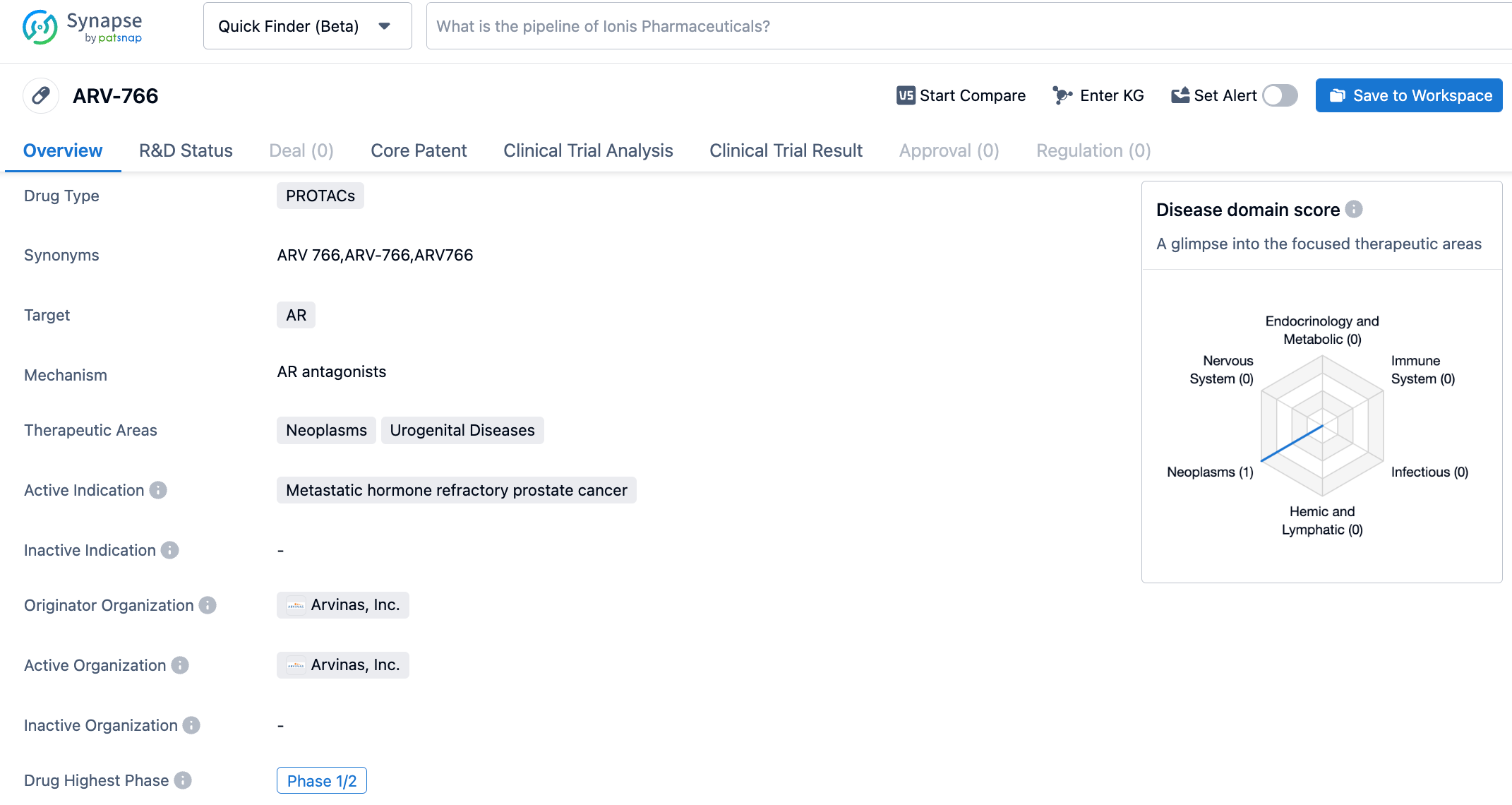
ARV-766, the second-generation oral AR degrader developed by Arvinas, has broader genotype coverage and better stereochemical stability than its predecessor ARV-110. All AR LBD mutations, especially AR L702H, increase in frequency over time. About 25% of tumors have these mutations after initial treatment with new hormone drugs (NHAs) such as enzalutamide or abiraterone.
Extended follow-up data from the Phase 1/2 clinical trials of ARV-110 showed radiographic progression-free survival (rPFS) of 11.1 months in the mCRPC patients cohort with AR T878X/H878Y mutations (AR 878/875; T878X=T878A or T878S) in the absence of AR L702H mutations. 54% of patients’ prostate-specific antigen (PSA) levels decreased by ≥50% (PSA50). In patients with any AR LBD mutation (excluding L702H mutation), the rPFS of ARV-110 was 8.2 months, with 36% of patients achieving PSA50. However, only 8% of patients with AR L702H mutations achieved PSA50 after treatment with ARV-110.
The most recent clinical trial results showed that ARV-766 decreased the PSA levels by ≥50% in 42% of patients with AR ligand-binding domain (LBD) mutations. 3 out of 5 patients with AR L702H mutations achieved PSA50. Among these 3 cases of AR L702H mutation, all of them also showed concurrent T878/H875 mutations. In 4 RECIST-evaluable patients with tumors with AR LBD mutations, two observed partial remission (1 confirmed partial remission, 1 unconfirmed partial remission). ARV-766 is currently also in the Phase 2 clinical research stage.
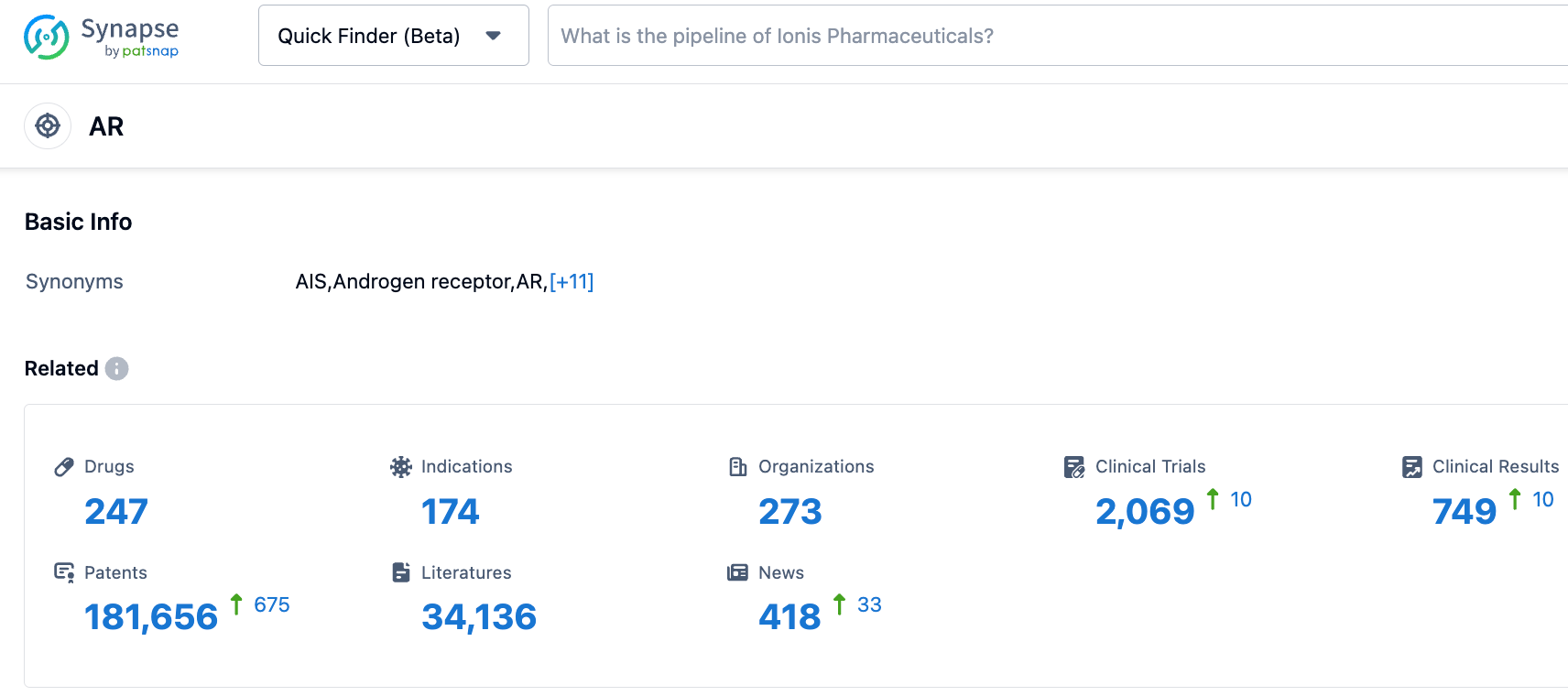
According to information disclosed by the synapse database, as of October 25, 2023, there are 247 in-developed drugs targeting AR, including 174 indications, 273 research institutions involved, 2068 related clinical trials, and as many as 181,693 patents. ARV-766 has shown signs of efficacy in late-stage cancer patients and has good tolerance. It is expected to break through the unmet medical needs of castration-resistance and castration-sensitive prostate cancer.