Benzydamine Hydrochloride Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Benzydamine Hydrochloride's R&D Progress
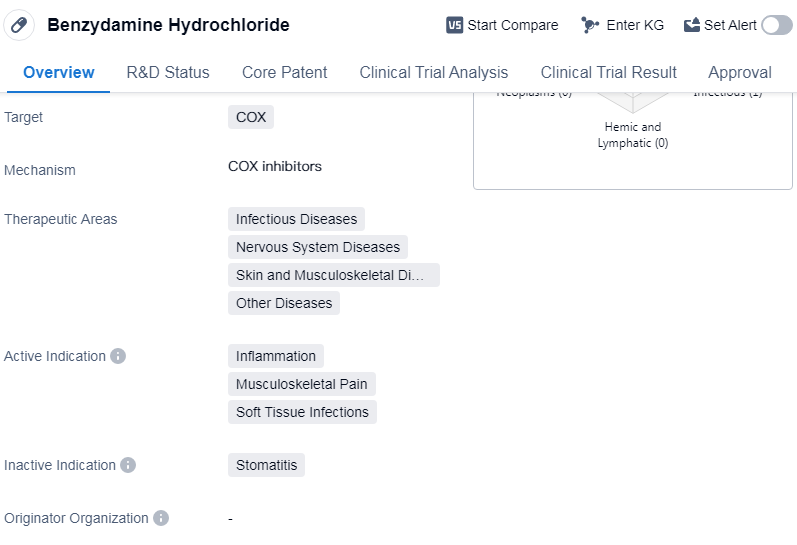
Benzydamine Hydrochloride is a small molecule drug that belongs to the class of nonsteroidal anti-inflammatory drugs (NSAIDs). It primarily targets the enzyme cyclooxygenase (COX), which is involved in the production of inflammatory mediators.
Benzydamine Hydrochloride has been approved for use in various therapeutic areas, including infectious diseases, nervous system diseases, skin and musculoskeletal diseases, and other diseases. It is primarily indicated for the treatment of inflammation, musculoskeletal pain, and soft tissue infections.
The highest phase of development for Benzydamine Hydrochloride is approved, indicating that it has successfully completed clinical trials and has been granted regulatory approval for marketing and use. This approval status applies globally.
Benzydamine Hydrochloride received its first approval in the United Kingdom in March 1980. This suggests that it has been available for clinical use for several decades and has established a track record of safety and efficacy.
Benzydamine Hydrochloride is regulated as an orphan drug. Orphan drugs are medications that are developed to treat rare diseases or conditions that affect a small number of patients. The regulatory designation of orphan drug provides certain incentives and benefits to the pharmaceutical company developing the drug, such as extended market exclusivity and financial incentives.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Benzydamine Hydrochloride: COX inhibitors
COX inhibitors are a type of medication that inhibit the activity of cyclooxygenase (COX) enzymes. These enzymes play a crucial role in the production of prostaglandins, which are hormone-like substances involved in various physiological processes, including inflammation, pain, and fever. By inhibiting COX enzymes, COX inhibitors reduce the production of prostaglandins, leading to anti-inflammatory, analgesic (pain-relieving), and antipyretic (fever-reducing) effects.
From a biomedical perspective, COX inhibitors are commonly used in the treatment of inflammatory conditions such as arthritis, as well as for pain management. They can help alleviate pain, reduce inflammation, and improve joint mobility in individuals with arthritis. Additionally, COX inhibitors are sometimes used as antipyretics to lower fever in certain situations.
There are two main types of COX inhibitors: nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors. NSAIDs, such as aspirin and ibuprofen, inhibit both COX-1 and COX-2 enzymes, while selective COX-2 inhibitors, such as celecoxib, specifically target COX-2 enzymes. The selectivity of COX-2 inhibitors helps minimize some of the side effects associated with traditional NSAIDs, particularly gastrointestinal complications.
It's important to note that while COX inhibitors can be effective in managing pain and inflammation, they may also have potential side effects, such as gastrointestinal bleeding, cardiovascular risks, and kidney problems. Therefore, it is crucial to use these medications under the guidance of a healthcare professional and follow the prescribed dosage and duration.
Drug Target R&D Trends for Benzydamine Hydrochloride
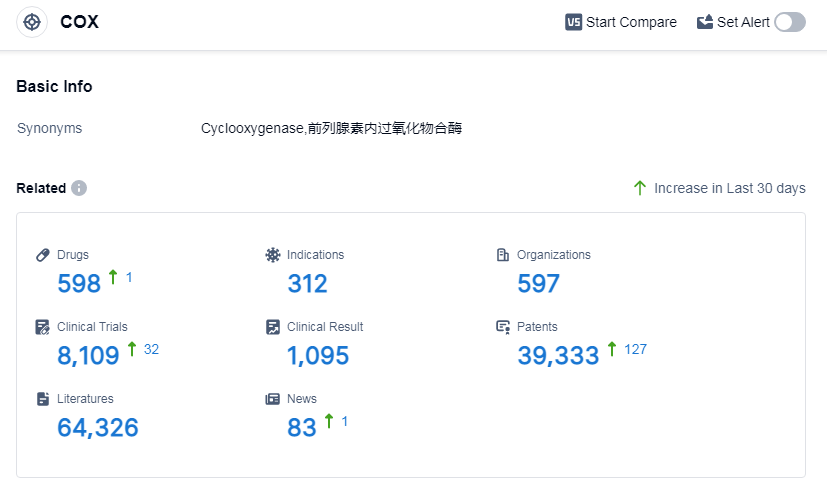
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 598 COX drugs worldwide, from 597 organizations, covering 312 indications, and conducting 8109 clinical trials.
The analysis of the target COX reveals that GSK Plc, Viatris Inc., and Pfizer Inc. are the companies growing the fastest under this target. These companies have made significant progress in the development of drugs targeting COX, with GSK Plc having the highest number of approved drugs.
The most common indications for drugs targeting COX are pain, common cold, rheumatoid arthritis, and osteoarthritis. These indications highlight the importance of developing effective treatments for these conditions.
Small molecule drugs are the most rapidly progressing drug type under the target COX. This indicates a focus on developing innovative small molecule drugs for the treatment of various indications.
China, the United States, Japan, and the European Union are the countries/locations that are developing the fastest under the target COX. China, in particular, has shown significant progress in the development of approved drugs, indicating its growing presence in the pharmaceutical industry.
Overall, the competitive landscape for target COX is dynamic, with multiple companies and countries actively involved in research and development. The future development of target COX holds potential for the advancement of treatments for various indications, particularly in the areas of pain, rheumatoid arthritis, and osteoarthritis.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Benzydamine Hydrochloride is a small molecule drug that targets COX and is approved for use in various therapeutic areas, including infectious diseases, nervous system diseases, skin and musculoskeletal diseases, and other diseases. It is primarily indicated for the treatment of inflammation, musculoskeletal pain, and soft tissue infections. The drug received its first approval in the United Kingdom in 1980 and is regulated as an orphan drug.