Mapping the Evolution of the Competitive Landscape of Mirvetuximab Soravtansine
A significant milestone was recently achieved in the evolution of the ovarian cancer therapeutic landscape, with China's National Medical Products Administration (NMPA) issuing a Notice of Acceptance to Huadong Medicine Co., Ltd. This pharmaceutical giant and its collaborator, ImmunoGen, Inc., based in America, received acceptance for their Market Application for the groundbreaking drug, Mirvetuximab Soravtansine Injection.
Pertaining to the Sotuximab Monotherapy Injection
The key highlight of this injection is its first-in-class characterization, being an ADC drug, that is a joint venture of Huadong Medicine and ImmunoGen. The unique component the drug targets is the Folate Receptor α (FRα), which is a cell surface protein, responsible for the aggressive multiplication of ovarian cancer cells. The sophisticated construction of this drug incorporates FRα binding antibodies, cleavable linkers and the maytansinoid DM4 substance.
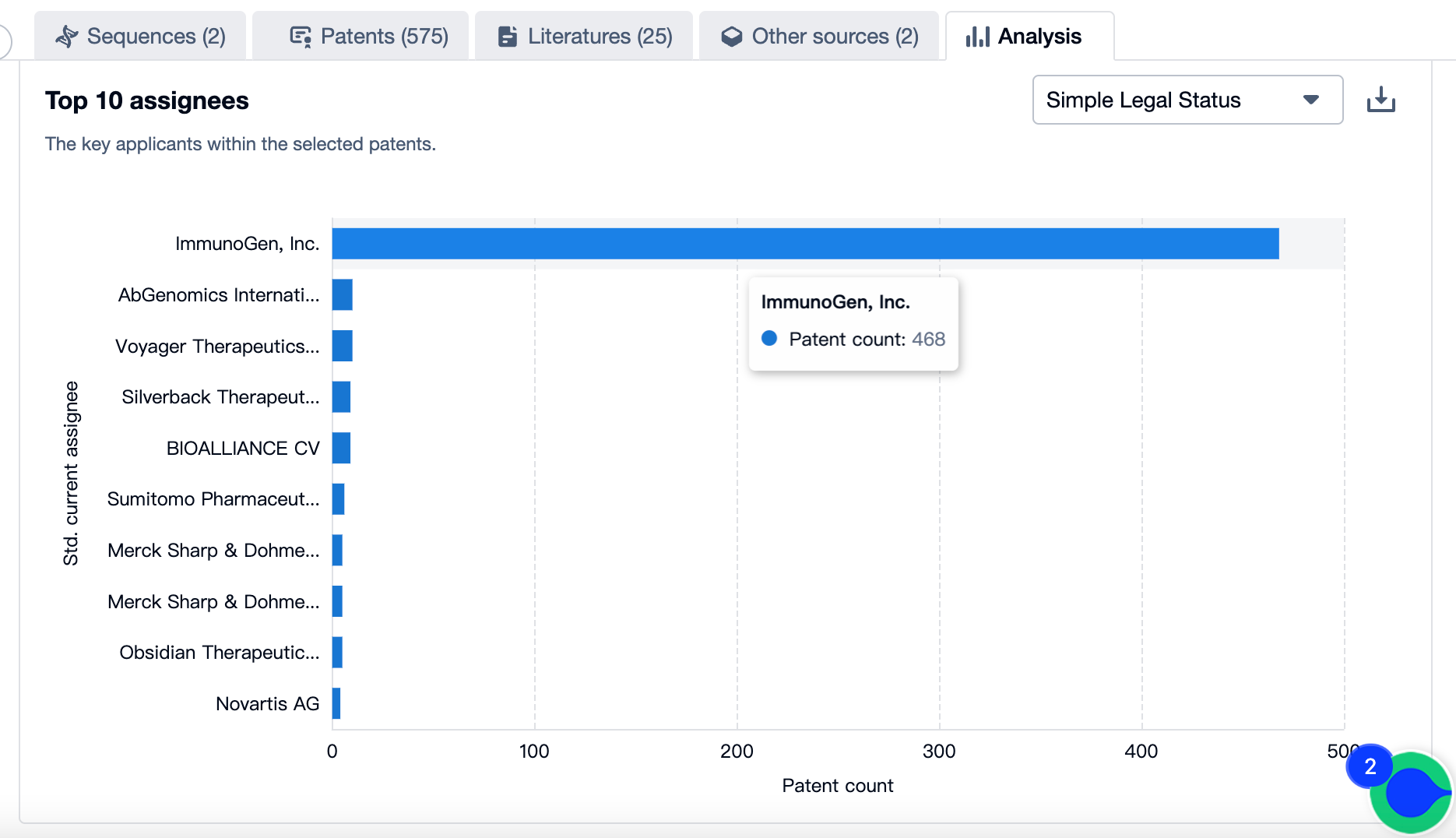
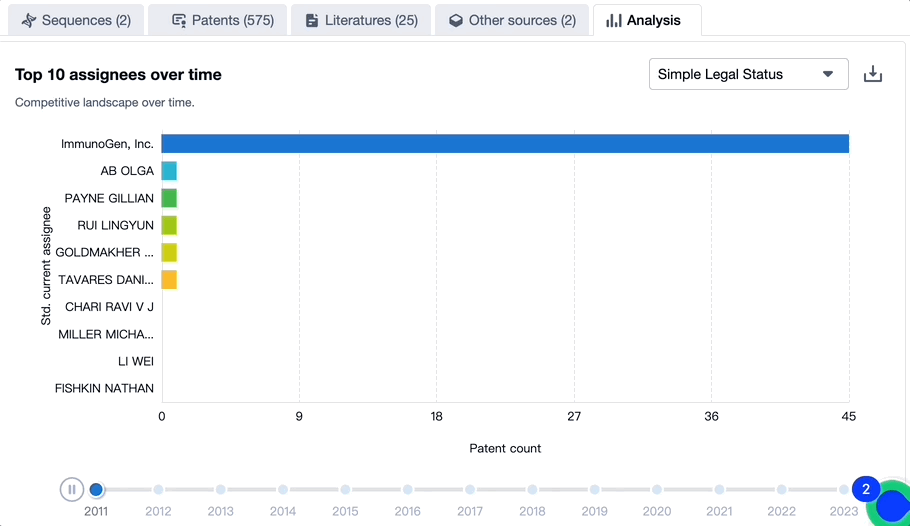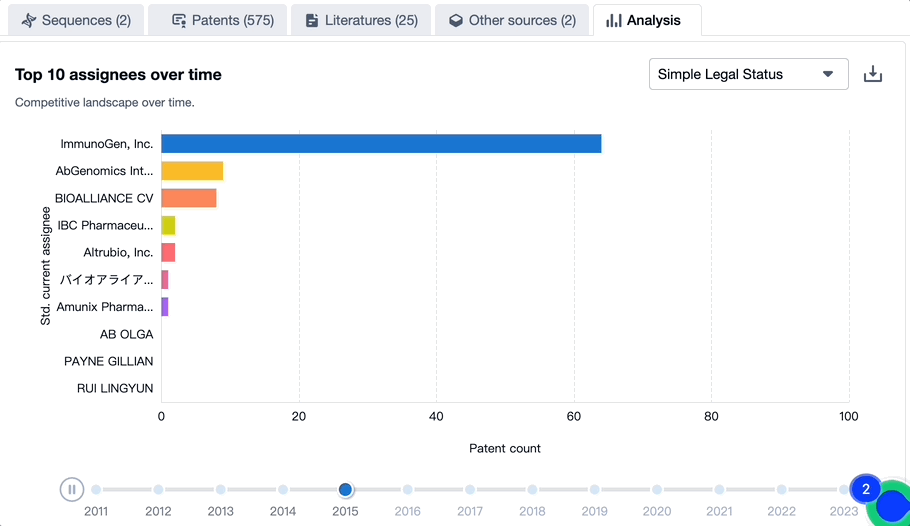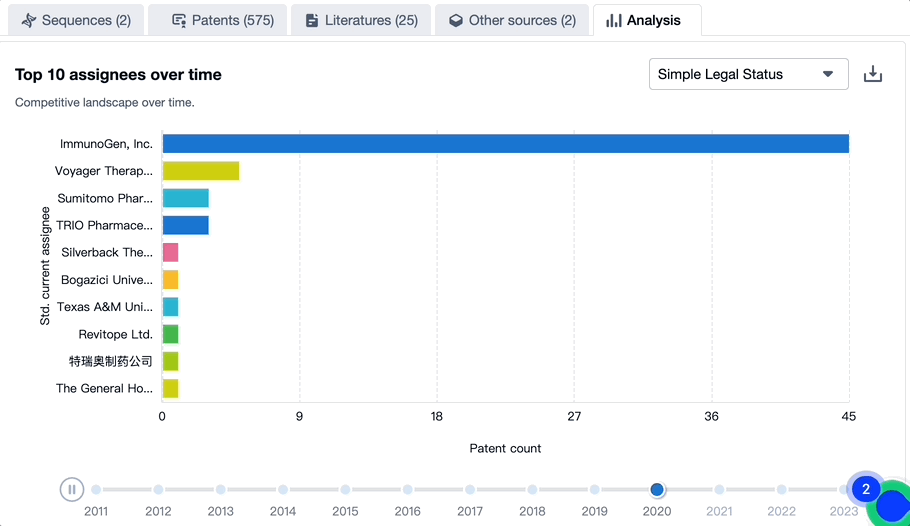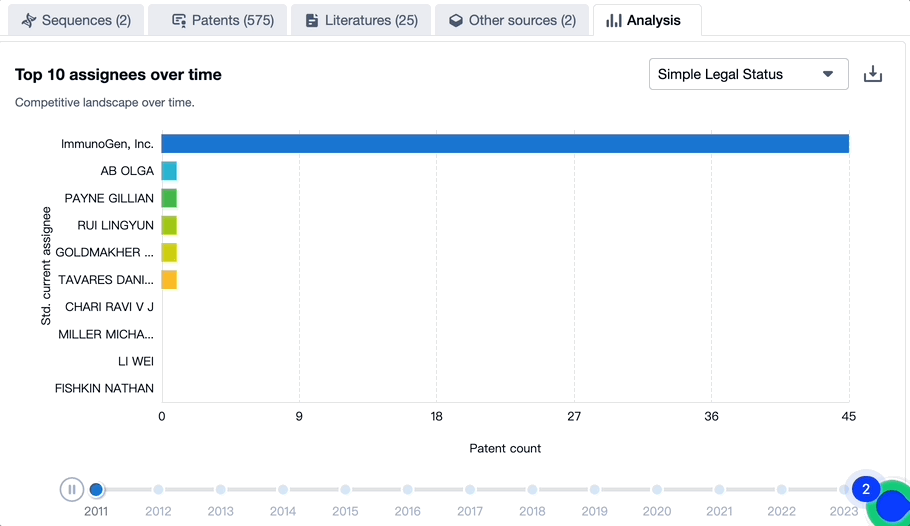
Patenting Landscape of Mirvetuximab Soravtansine: Top 10 Assignees
Source, Patsnap Bio
Evolution of the Layout of Top 10 Patent Assignees over the years, the Competitive landscape over time
In mapping the evolutionary trajectory of the patenting landscape pertaining to Mirvetuximab Soravtansine, it is critical to analyze the strategic approach of the top 10 assignees. There is also a dynamic shift in the competition blueprint that is dictated by the progression of time.
Source, Patsnap Bio
Requisite Steps for Accessing Comprehensive Sequence Patent Details about Mirvetuximab Soravtansine
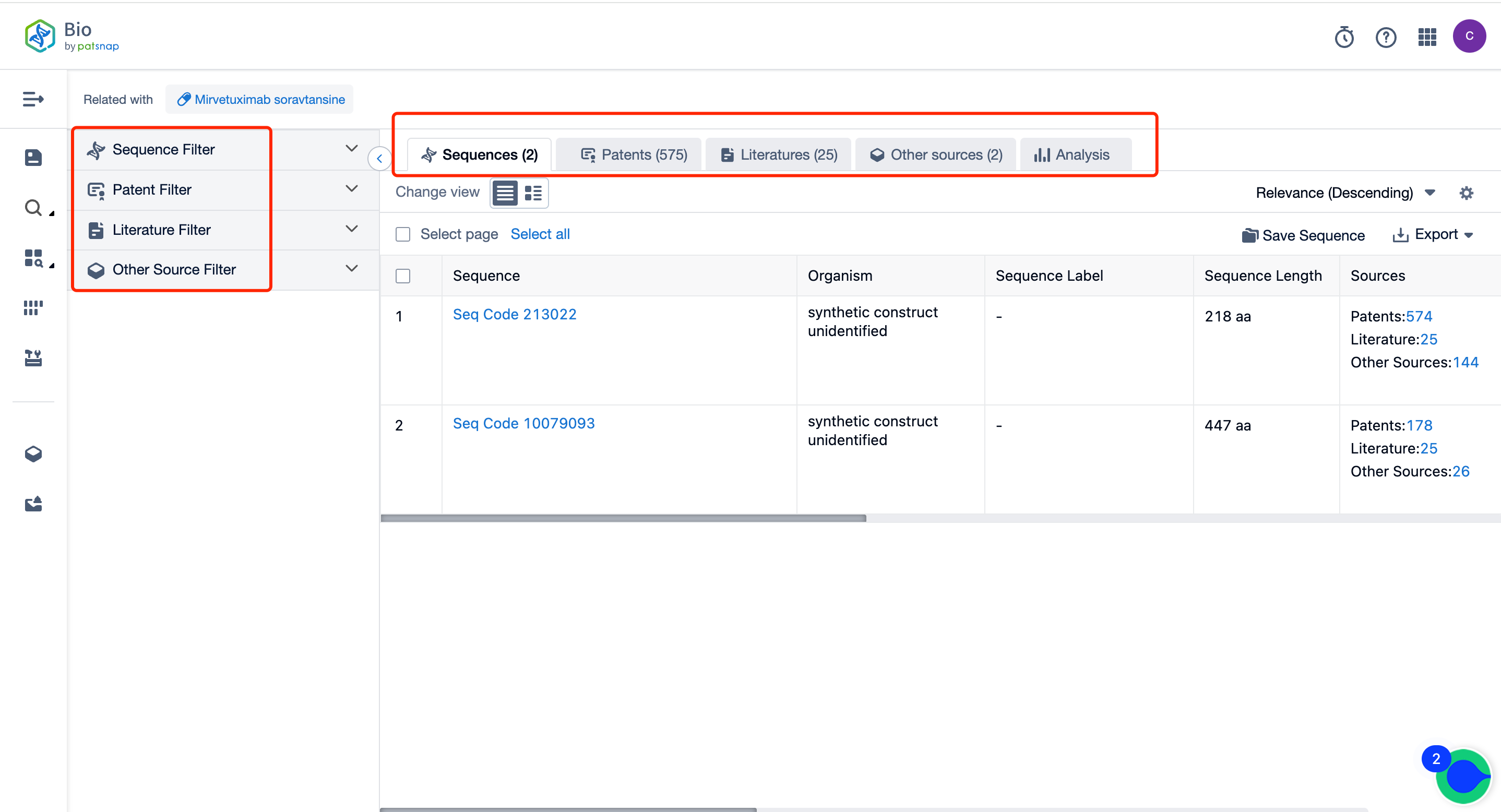
Interested parties could utilize the Patsnap Bio Sequence Database, which is easily accessible by creating a free account. On the home page, you can either enter the molecular sequence of Mirvetuximab Soravtansine into the "Common Search" bar or simply key in the drug's name in the "Drug/Gene Index." A single search effort would provide an array of information, such as: sequence specifics, related literature, patent particulars, relevant additional data, and a visual display of the drug’s competitive patent landscape.
For an enhanced search experience, the interface provides a robust filtering mechanism, streamlining your desired details. Delving into each data entry further provides access to a rich collection of related data, along with efficient, user-friendly features to augment your research endeavors.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.