Unleashing the Power of Benazepril Hydrochloride: A Comprehensive Review on R&D Breakthroughs
Benazepril Hydrochloride's R&D Progress
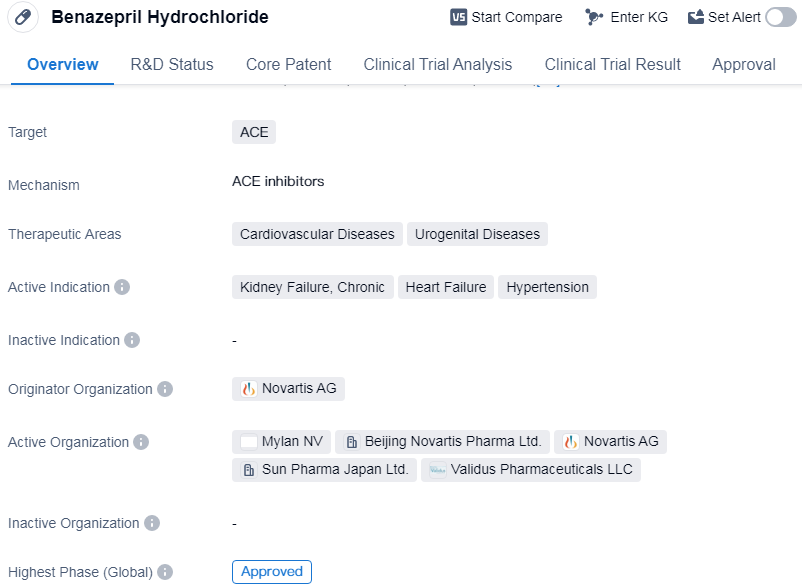
Benazepril Hydrochloride is a small molecule drug that targets ACE (angiotensin-converting enzyme). It is primarily used in the treatment of cardiovascular diseases and urogenital diseases. The active indications for this drug include kidney failure, chronic heart failure, and hypertension.
The drug was first approved globally in January 1989 and has since been widely used in the pharmaceutical industry.
The originator organization of this drug is Novartis AG, a renowned pharmaceutical company known for its contributions to the healthcare industry. Novartis AG has played a significant role in the development and commercialization of Benazepril Hydrochloride.
Benazepril Hydrochloride's highest phase of development, is approved globally. This suggests that it has successfully completed all necessary clinical trials and regulatory requirements, demonstrating its safety and efficacy in treating the indicated conditions.
Benazepril Hydrochloride's therapeutic areas including cardiovascular diseases and urogenital diseases, highlight its potential to address a range of medical conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Benazepril Hydrochloride: ACE inhibitors
ACE inhibitors are a type of medication commonly used in the field of biomedicine to treat various cardiovascular conditions. ACE stands for angiotensin-converting enzyme, which plays a crucial role in regulating blood pressure by converting angiotensin I to angiotensin II. Angiotensin II is a potent vasoconstrictor, meaning it narrows blood vessels and increases blood pressure.
By inhibiting the action of ACE, ACE inhibitors prevent the conversion of angiotensin I to angiotensin II, resulting in the relaxation and dilation of blood vessels. This helps to lower blood pressure and improve blood flow throughout the body. Additionally, ACE inhibitors also reduce the production of aldosterone, a hormone that promotes fluid retention, further aiding in blood pressure control.
ACE inhibitors are commonly prescribed for conditions such as hypertension (high blood pressure), heart failure, and certain kidney diseases. They are often used as a first-line treatment due to their effectiveness and relatively low risk of side effects. Some examples of ACE inhibitors include lisinopril, enalapril, and ramipril.
It is important to note that ACE inhibitors may have certain side effects, including a persistent dry cough, dizziness, and changes in kidney function. Therefore, it is essential to consult with a healthcare professional before starting or adjusting any medication regimen involving ACE inhibitors.
Drug Target R&D Trends for Benazepril Hydrochloride
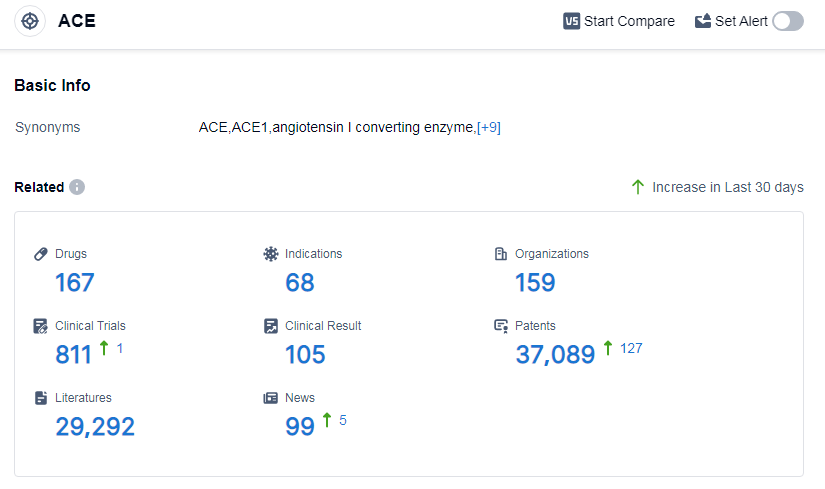
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 167 ACE drugs worldwide, from 159 organizations, covering 68 indications, and conducting 811 clinical trials.
The analysis of the target ACE reveals a competitive landscape with companies like Les Laboratoires Servier SAS, CHIESI Farmaceutici SpA, and Viatris Inc. leading the way in R&D progress. The most common indications for drugs under this target are hypertension, heart failure, and essential hypertension, indicating a focus on cardiovascular health. Small molecule drugs dominate the drug type analysis, with biosimilars showing intense competition. The European Union, China, the United States, and Japan are the fastest-developing regions, with notable progress in China's pharmaceutical industry. Overall, the target ACE presents opportunities for further research and development, particularly in addressing cardiovascular diseases.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Benazepril Hydrochloride is a small molecule drug that targets ACE and is primarily used in the treatment of cardiovascular diseases and urogenital diseases. It has been approved globally since 1989, with Novartis AG being the originator organization. The drug's active indications include kidney failure, chronic heart failure, and hypertension. Its approval status and therapeutic areas make it a valuable asset in the pharmaceutical industry, addressing critical medical conditions and improving patient outcomes.