Avalo Raises $185M for IL-1β Antibody Through Private Funding
Avalo Therapeutics, Inc. has announced the procurement of a monoclonal antibody targeting IL-1β, designated as AVTX-009, which is prepared to enter Phase 2 trials. This was achieved through the takeover of the private entity AlmataBio, Inc. In step with this acquisition, Avalo has finalized a deal regarding the issuance of preferred shares and warrants in a private funding round. This round was spearheaded by Commodore Capital and TCGX, with contributions coming from a group of investors including BVF Partners, Deep Track Capital, OrbiMed, Petrichor, and RA Capital Management.
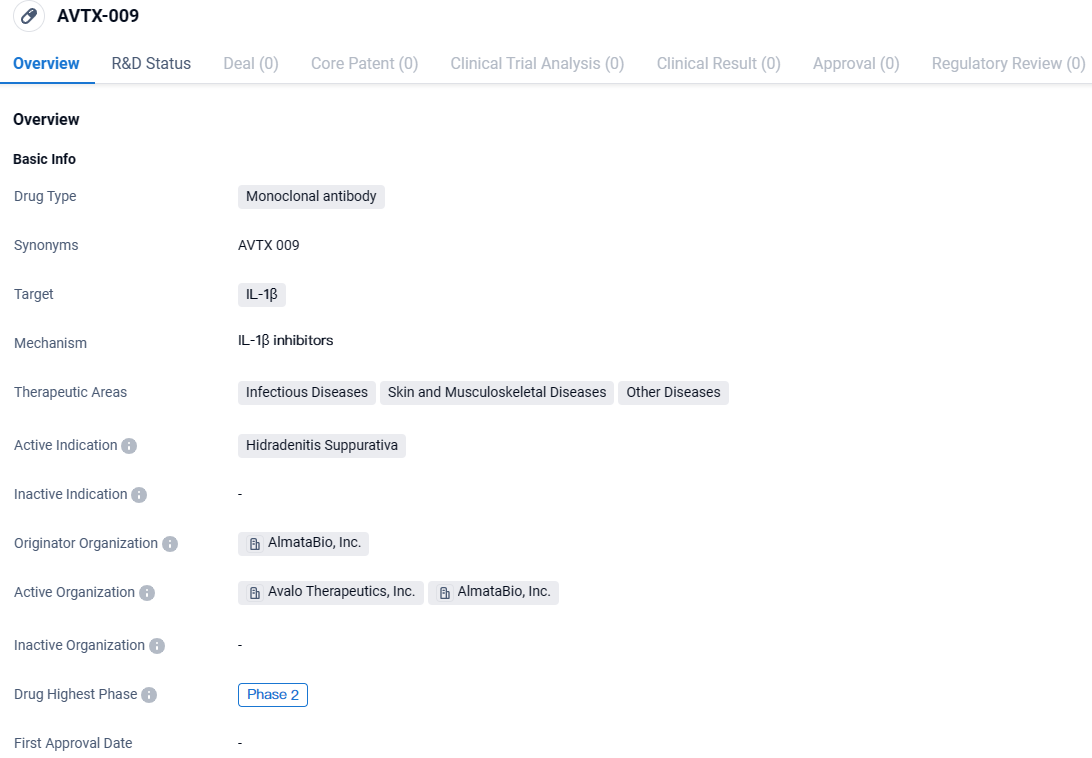
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
A series of private financial injections are poised to culminate in total revenues reaching up to a ceiling of $185 million, with the initial cash infusion pegged at $115.6 million. Post computation of the anticipated expenses linked to the private funding pursuit and AlmataBio's integration, Avalo foresees its clean initial gains to be in the neighborhood of $105 million. The financial endeavor is slated for completion on March 28, 2024, hinging on the accomplishment of standard finalizing provisions.
Avalo is charting a course towards the advancement of AVTX-009 for the ailment hidradenitis suppurativa (HS). Projections place the unveiling of pivotal Phase 2 trial outputs for HS in the year 2026, with the initial investment poised to underpin corporate functionality extending into 2027, covering this pivotal data unveiling. Beyond HS, Avalo's strategy includes expanding AVTX-009's therapeutic use to address at least one additional persistent inflammatory disorder.
“It's with great anticipation that we declare the procurement of AVTX-009 and the corresponding capital arrangement reaching up to $185 million. Committed to addressing inflammatory maladies, the team at Avalo is energized about embarking on a Phase 2 trial directed at HS, a condition with a pressing necessity for innovative treatments. Backed by recent findings that validate the role of IL-1β suppression in HS, we are optimistic about AVTX-009’s prospects for success, a product originally curated by Eli Lilly.
Anticipating the immense market potential for HS treatments, AVTX-009 stands out with the promise of leading its class and indication, banking on its precise targeting, durability, and strength, possibly heralding robust effectiveness and user-friendly administration,” proclaimed Dr. Garry A. Neil, Avalo's CEO and Chairman of the Board. “The financial surety to the awaited data release point bolsters our pursuit, and we are grateful for the endorsement from an impressive cadre of investors.”
Dr. Neil elaborated, “Gratitude is due to Patrick Crutcher and his squad at AlmataBio for steering this high-caliber molecule to the forefront. Crutcher's biography reflects a string of biotechnological initiatives and uncovering promising ventures. His collaboration and acumen have been immensely valued.”
Offering his perspective, Patrick J. Crutcher, the ex-CEO of AlmataBio, commented, “We are heartened by Avalo's recognition of the transformative capability encapsulated in AVTX-009 for patients combatting inflammatory disorders. AlmataBio's inception rested on the notion of scouting, securing, and fast-tracking pivotal therapeutic innovations, and the savvy AlmataBio collective has admirably fulfilled this pledge. We eagerly anticipate AVTX-009's progression into a Phase 2 trial for HS curated by Dr. Neil.”
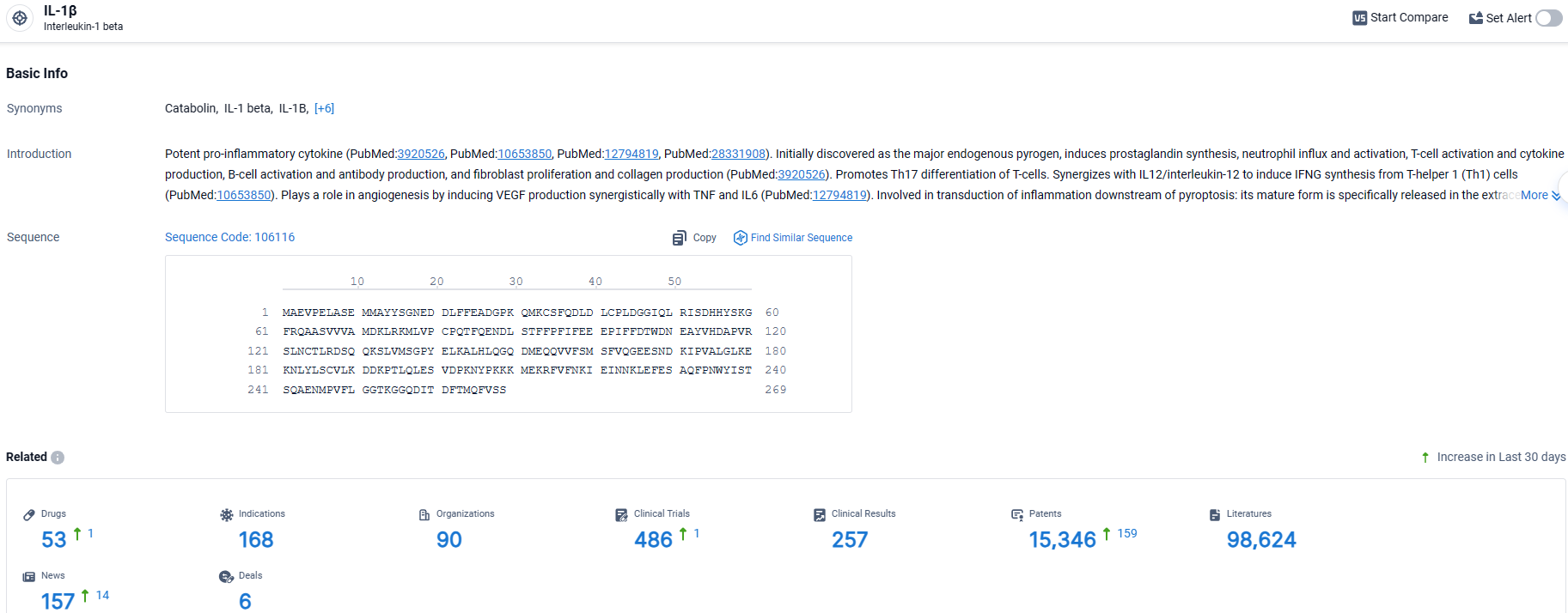
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 29 2024, there are 53 investigational drugs for the IL-1β target, including 168 indications, 90 R&D institutions involved, with related clinical trials reaching 486, and as many as 15346 patents.
AVTX-009 targets IL-1β and is being developed for the treatment of Hidradenitis Suppurativa, a chronic inflammatory skin condition. The drug is currently in Phase 2 of clinical trials, indicating that it has progressed beyond initial safety testing and has shown potential efficacy.