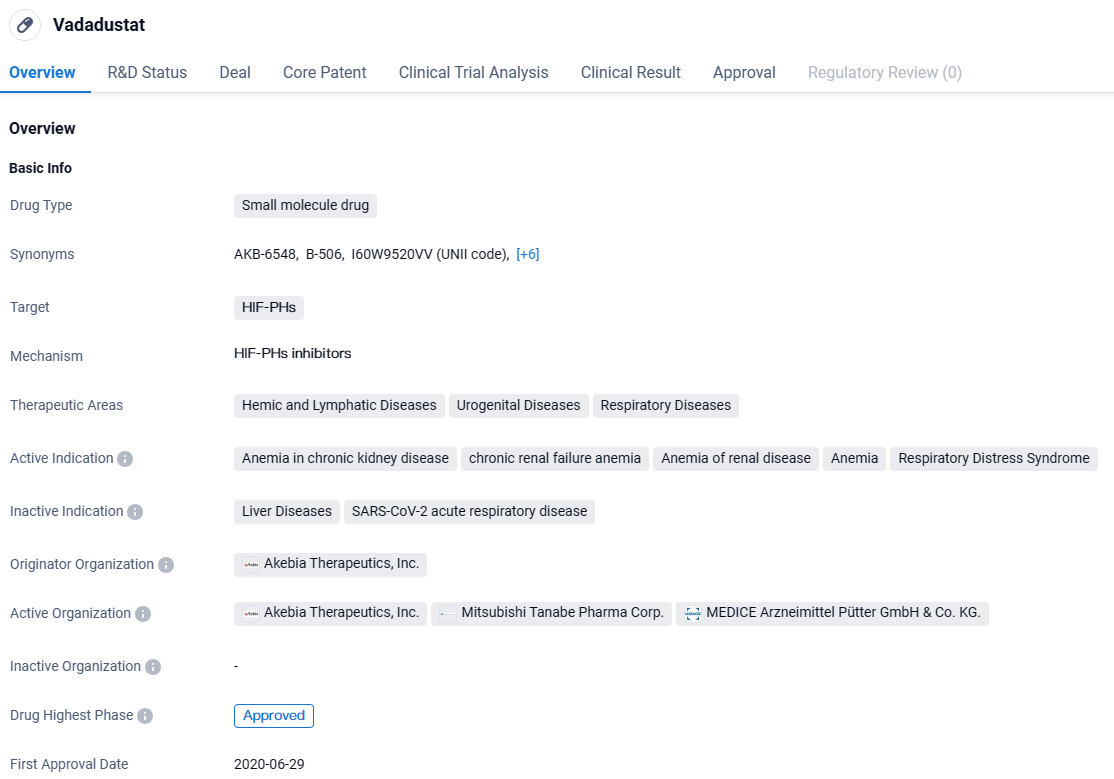
Vafseo Pill Approved for Adult Dialysis-Related Anemia in the US
Akebia Therapeutics, Inc., a company specializing in biopharmaceuticals and dedicated to enhancing the well-being of individuals suffering from renal conditions, recently declared that the U.S. Food and Drug Administration has granted approval for the use of Vafseo® (vadadustat) as a tablet formulation to manage anemia related to chronic kidney disease in adult patients who have undergone dialysis treatment for a minimum duration of three months.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Vafseo, administered once daily by mouth, is an innovative HIF-PH inhibitor that boosts the body’s natural response to low oxygen levels by increasing autonomous erythropoietin output, effectively combating anemia. Currently, Vafseo has obtained clearance in a total of 37 nations.
John P. Butler, Akebia's CEO, expressed his enthusiasm regarding the authorization of Vafseo in the United States, offering a fresh therapeutic avenue for the sizable population of American dialysis patients afflicted by CKD-related anemia. "Achieving this significant regulatory milestone underscores Akebia's unwavering focus on the renal community," stated Butler. The company's commitment is expected to be instrumental in the smooth introduction of Vafseo, potentially setting a novel oral care benchmark for patients undergoing dialysis.
Vafseo's green light for addressing adult anemia stemming from CKD in patients who are at least three months into their dialysis treatment draws support from the compelling results observed within the INNO2VATE studies, coupled with insights from the follow-up of its market use in Japan, where it debuted in August 2020.
Founder and President of the Renal Support Network, Lori Hartwell, has battled with kidney disease from childhood. She conveyed optimism regarding Vafseo's potential impact on adults with dialysis-dependent chronic kidney disease anemia by emphasizing that anemia severely exerts a toll on day-to-day well-being. The advent of new treatment pathways for combating anemia is highly encouraging for affected individuals.
With plans to market Vafseo across the U.S., Akebia leverages a skilled commercial force with significant renal expertise in tandem with a partnership with CSL Vifor, renowned for introducing groundbreaking treatments to U.S. dialysis centers. Strategic market introduction activities will align with the sanctioned indications to position Vafseo as a leading oral care alternative for the adult dialysis demographic.
“We owe a debt of gratitude to the patients, medical professionals, researchers, and coordinators who contributed to our studies and helped obtain this crucial endorsement," Mr. Butler remarked. This landmark achievement is the fruit of persistent efforts from the Akebia team and its partners, all dedicated to improving the quality of life for individuals affected by renal diseases.
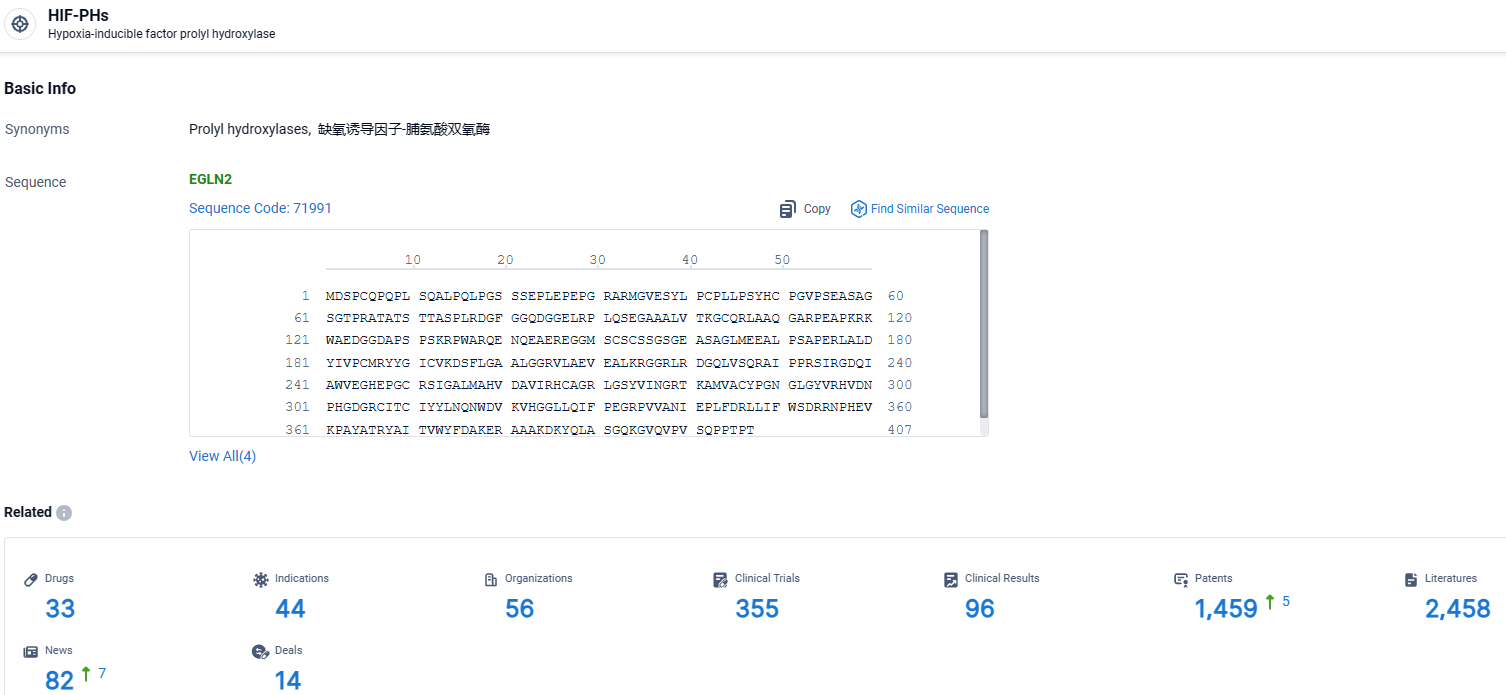
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 29, 2024, there are 33 investigational drugs for the HIF-PHs target, including 44 indications, 56 R&D institutions involved, with related clinical trials reaching 355, and as many as 1459 patents.
Vadadustat is a small molecule drug that targets HIF-PHs and shows potential in treating various diseases, particularly anemia in chronic kidney disease. The drug has reached the highest phase of development globally and has received its first approval in Japan. Ongoing clinical trials in China indicate the company's interest in expanding the drug's availability to other markets.