Pharmaceutical Insights: Baloxavir Marboxil's R&D Progress and its Mechanism of Action on Drug Target
Baloxavir Marboxil's R&D Progress
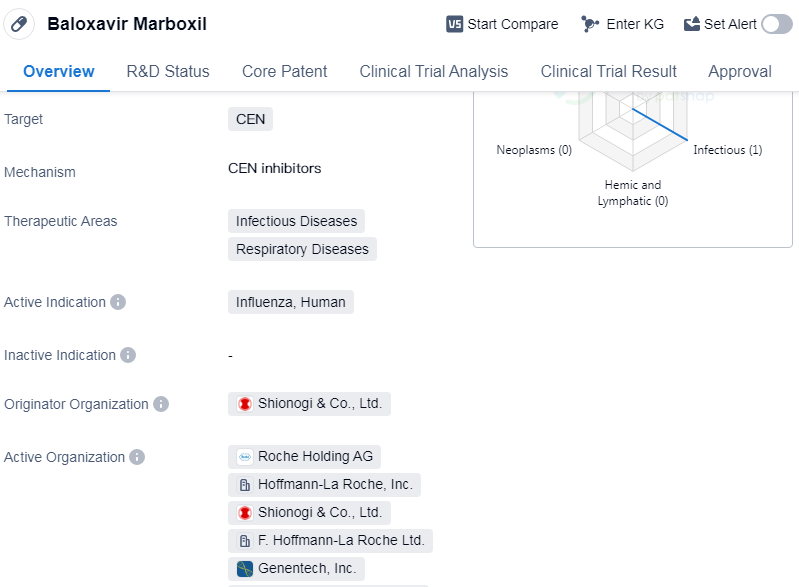
Baloxavir marboxil is a small molecule drug that targets the CEN protein. It is primarily used in the treatment of infectious diseases and respiratory diseases. The drug is specifically indicated for the treatment of influenza in humans.
The originator organization of baloxavir marboxil is Shionogi & Co., Ltd., a pharmaceutical company based in Japan. The drug has received approval in multiple countries, with its highest phase of development being approved globally. It was first approved in Japan in February 2018.
Baloxavir marboxil has been granted priority review status and is considered an overseas new drug urgently needed in clinical settings. This suggests that the drug has demonstrated significant potential in addressing unmet medical needs and has undergone an expedited review process.
The approval of baloxavir marboxil marks an important milestone in the treatment of influenza. As a small molecule drug, it has the potential to offer a more targeted and effective approach to combating the virus. By targeting the CEN protein, baloxavir marboxil disrupts the replication process of the influenza virus, thereby reducing the severity and duration of symptoms.
Infectious diseases, including influenza, pose a significant global health burden. The approval of baloxavir marboxil provides healthcare professionals with an additional tool in their arsenal to combat this infectious disease. Furthermore, its approval in multiple countries highlights the global recognition of its therapeutic potential.
The regulatory status of baloxavir marboxil, including priority review and its designation as an overseas new drug urgently needed in clinical settings, underscores the importance of this drug in addressing unmet medical needs. These designations facilitate a faster and more streamlined approval process, ensuring that patients have timely access to this potentially life-saving treatment.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Baloxavir Marboxil: CEN inhibitors
CEN inhibitors refer to a class of compounds or drugs that inhibit the activity of centromere-associated proteins. Centromeres are specialized regions of chromosomes that play a critical role in cell division and chromosome segregation. The proteins associated with centromeres, known as centromere-associated proteins, are involved in maintaining the structure and function of centromeres.
From a biomedical perspective, CEN inhibitors can be used as therapeutic agents to target and disrupt the function of centromeres in various disease conditions. For example, aberrant centromere function has been implicated in cancer, where chromosomal instability and abnormal chromosome segregation are observed. By inhibiting the activity of centromere-associated proteins, CEN inhibitors can potentially interfere with cancer cell division and promote cell death.
It is important to note that CEN inhibitors are still under investigation and development, and their specific mechanisms of action and potential therapeutic applications may vary depending on the specific compound or drug being studied.
Drug Target R&D Trends for Baloxavir Marboxil
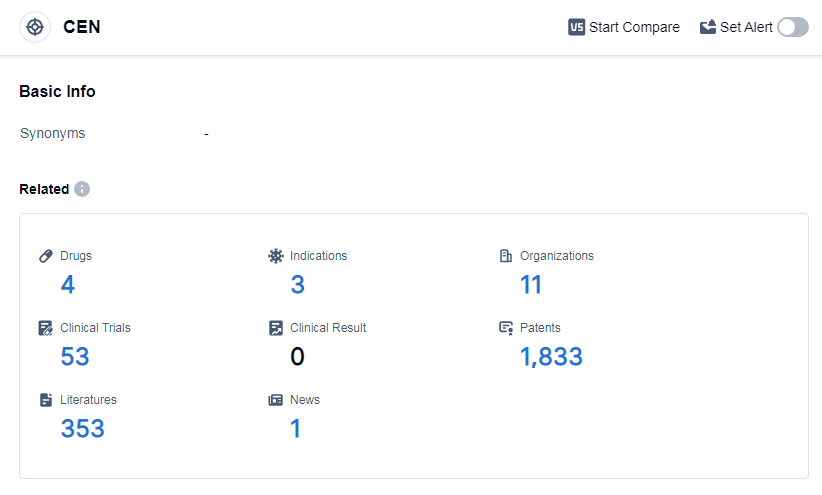
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 4 CEN drugs worldwide, from 11 organizations, covering 3 indications, and conducting 53 clinical trials.
The current competitive landscape of target CEN shows that Roche Holding AG, Shionogi & Co., Ltd., TaiGen Biopharmaceuticals Holdings Ltd., Andikang (Wuxi) Biotechnology Co., Ltd., Joincare Pharmaceutical Group Industry Co., Ltd., and ChemRar High-Tech Center are the companies growing fastest. Roche Holding AG has the highest stage of development with drugs in the approved, phase 3, and inactive phases. The drugs under the current target CEN have been approved for indications related to influenza. Small molecule drugs are progressing rapidly, with one drug in the approved phase and one in the preclinical phase. China, along with other countries such as the European Union, United States, and Japan, is developing rapidly under the current target. The progress in China indicates a significant contribution to the research and development efforts. Overall, the target CEN shows promising growth potential in the pharmaceutical industry, with active R&D progress and a focus on influenza-related indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Baloxavir Marboxil is a small molecule drug that targets the CEN protein and is primarily used in the treatment of influenza in humans. Its approval in multiple countries, including Japan, highlights its global recognition and therapeutic potential. The drug's regulatory status as a priority review and overseas new drug urgently needed in clinical settings further emphasizes its importance in addressing unmet medical needs.