Betamethasone Sodium Phosphate: Detailed Review of its Transformative R&D Success
Betamethasone Sodium Phosphate's R&D Progress
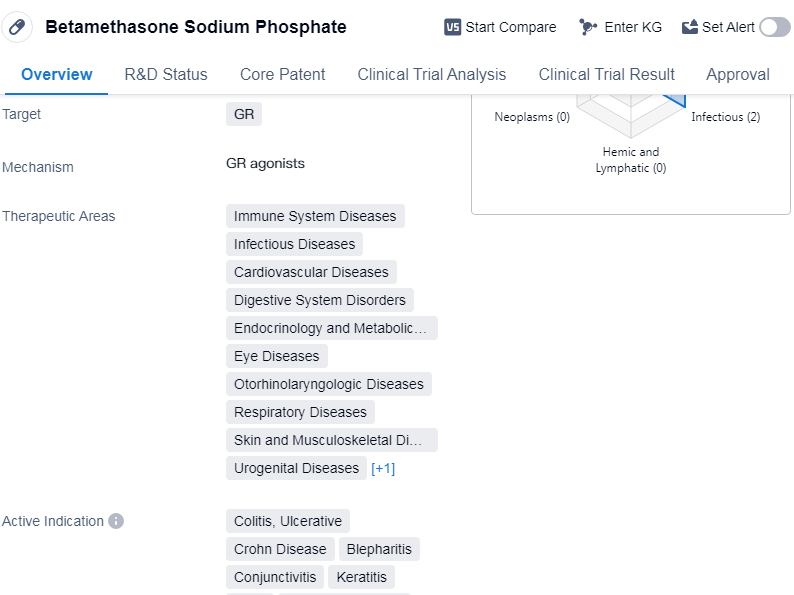
Betamethasone Sodium Phosphate is a small molecule drug that primarily targets the glucocorticoid receptor (GR). It has been approved for use in various therapeutic areas, including immune system diseases, infectious diseases, cardiovascular diseases, digestive system disorders, endocrinology and metabolic diseases, eye diseases, otorhinolaryngologic diseases, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases.
The active indications for Betamethasone Sodium Phosphate include colitis, ulcerative, keratitis, otitis, collagen diseases,etc.
The drug was first approved in Japan in October 1964 and is currently in the highest phase of development, which is approved. The originator organization of Betamethasone Sodium Phosphate is GSK Plc.
Betamethasone Sodium Phosphate is a widely used drug due to its broad therapeutic areas and active indications. It is commonly prescribed for inflammatory conditions such as colitis, Crohn's disease, and rheumatoid arthritis. The drug's mechanism of action involves binding to the glucocorticoid receptor, which helps regulate immune responses and reduce inflammation.
In addition to its anti-inflammatory properties, Betamethasone Sodium Phosphate is also used in the treatment of various eye diseases, including blepharitis, conjunctivitis, and keratitis. It is effective in reducing ocular inflammation and treating eye infections.
Furthermore, the drug is utilized in the management of otorhinolaryngologic diseases such as otitis externa and otitis media. It helps alleviate symptoms associated with these conditions, including ear pain and inflammation.
Betamethasone Sodium Phosphate has been approved for use in multiple countries worldwide since its first approval in Japan in 1964. Its long history of use and widespread acceptance in the medical community highlight its efficacy and safety profile.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Betamethasone Sodium Phosphate: GR Agonists
GR agonists are a type of medication that activates the GR. The GR is a protein found in cells that binds to the hormone cortisol, which is a natural glucocorticoid produced by the adrenal glands. When GR agonists bind to the GR, they mimic the effects of cortisol and initiate a series of cellular responses.
From a biomedical perspective, GR agonists are commonly used in the treatment of various conditions, including inflammatory disorders, autoimmune diseases, and certain types of cancer. By activating the GR, these agonists can help regulate immune responses, reduce inflammation, and suppress the activity of the immune system. This can be beneficial in managing chronic inflammatory conditions and preventing the immune system from attacking healthy tissues.
It's important to note that while GR agonists can be effective in treating certain conditions, long-term use or high doses of these medications can have side effects. These may include increased risk of infections, impaired wound healing, osteoporosis, and metabolic changes. Therefore, the use of GR agonists should be carefully monitored by healthcare professionals to ensure the benefits outweigh the potential risks.
Drug Target R&D Trends for Betamethasone Sodium Phosphate
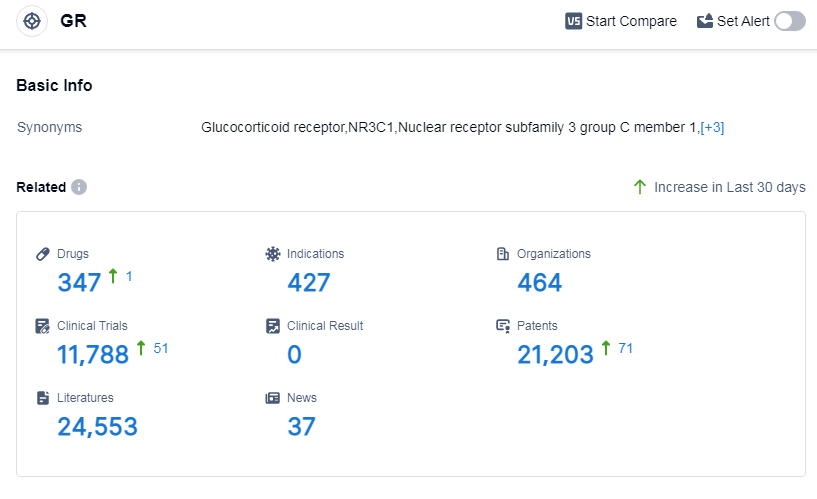
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 347 GR drugs worldwide, from 464 organizations, covering 427 indications, and conducting 11788 clinical trials.
The analysis of the target GR in the pharmaceutical industry reveals a competitive landscape with several companies demonstrating significant growth. Novartis AG, Shionogi & Co., Ltd., GSK Plc, and other organizations have shown strong R&D progress in this field. Indications such as eczema, asthma, and psoriasis have a high number of approved drugs, indicating active research in these areas.
Small molecule drugs dominate the drug type analysis, highlighting their prominence in the target GR field. However, other drug types such as hormones, ADC, monoclonal antibodies, and antisense oligonucleotides also show potential for innovative therapies.
The United States, China, and Japan lead in terms of approved drugs, with China's progress being particularly noteworthy. China's emergence as a major player in the target GR field signifies the shifting dynamics of global pharmaceutical research and development.
Overall, the target GR field presents significant growth opportunities for companies and researchers. The diverse pipeline of drugs, focusing on specific indications, and global development efforts indicate a promising future for advancements in gene regulation therapies.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Betamethasone Sodium Phosphate is a small molecule drug that targets the glucocorticoid receptor and is approved for various therapeutic areas and active indications. Its broad range of applications in immune system diseases, infectious diseases, cardiovascular diseases, and other conditions make it a valuable asset in the pharmaceutical industry.