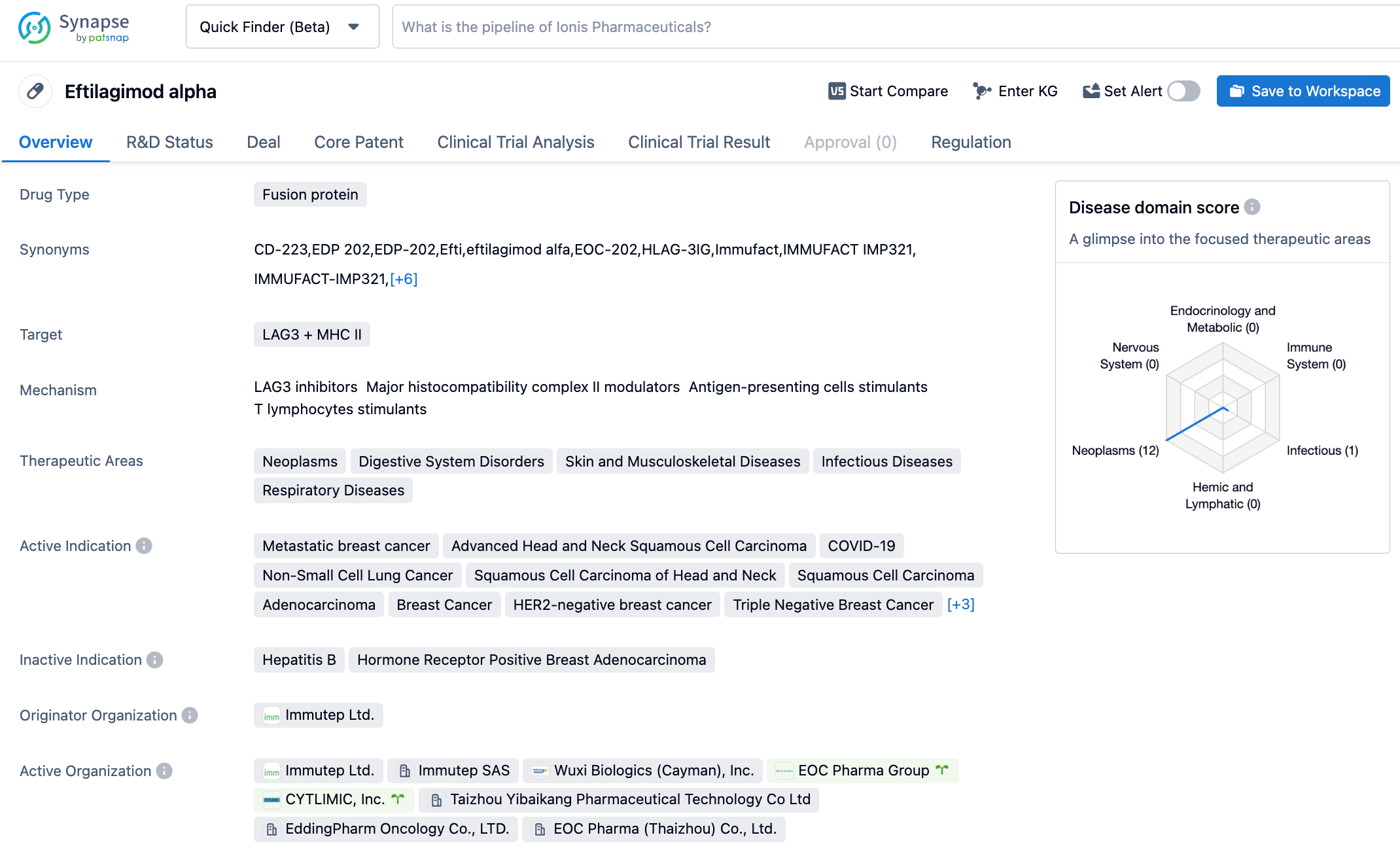
Immutep announces positive clinical results for two studies of its novel LAG-3 immunotherapy, eftilagimod alpha
Recently, Immutep presented an abstract at the 2023 European Society for Medical Oncology (ESMO) meeting, introducing its novel LAG-3 immunotherapy for treating cancer and autoimmune diseases, i.e, Eftilagimod Alpha (Efti), a LAG-3 fusion protein therapy, in combination with the PD-1 inhibitor pembrolizumab ± double chemotherapy, as first-line treatments for non-small cell lung cancer (NSCLC). The abstract features positive data from two clinical trials.
Eftilagimod Alpha is a soluble LAG-3 protein that can bind to subtype molecules of the Major Histocompatibility Complex II (MHC II), mediating the activation of Antigen-Presenting Cells (APC) and CD8 T cells. The stimulation of APC and subsequently recruited T cells by Eftilagimod Alpha could potentially reverse PD-1/PD-L1 resistance. LAG-3 protein can regulate the signaling pathway of T lymphocytes and Antigen presenting cells (APCs), playing a crucial role in adaptive immune responses. Soluble LAG-3, by binding with MHC II on the surface of APCs, can activate these APCs, leading to an increase and activation of cytotoxic CD8 positive T cells. Through this mechanism, soluble LAG-3 protein can enhance the immune response to cancer antigens. Previous research has shown that combining anti-LAG-3 antibodies with anti-PD-1 antibodies could potentially synergistically activate T cells.
The TACTI-002 trial evaluated the effect of a combined therapy of Efti and pembrolizumab as a first-line treatment for non-small cell lung cancer. Data till March 31, 2023, indicates that irrespective of PD-L1 expression, patients with non-small cell lung cancer can benefit from the combination of Efti and pembrolizumab. Specifically, the survival period for patients with a PD-L1 tumor proportion score (TPS) ≥50% reached 38.8 months, for those with a TPS of 1-49% was 23.4 months, and for those with a TPS ≥1% was 25.0 months. The INSIGHT-003 trial evaluated the impact of Efti combined with pembrolizumab and double chemotherapy as a first-line treatment for non-squamous NSCLC patients. Data till April 18, 2023, showed that the overall response rate for this triple therapy was 67%, and the disease control rate reached 91%.
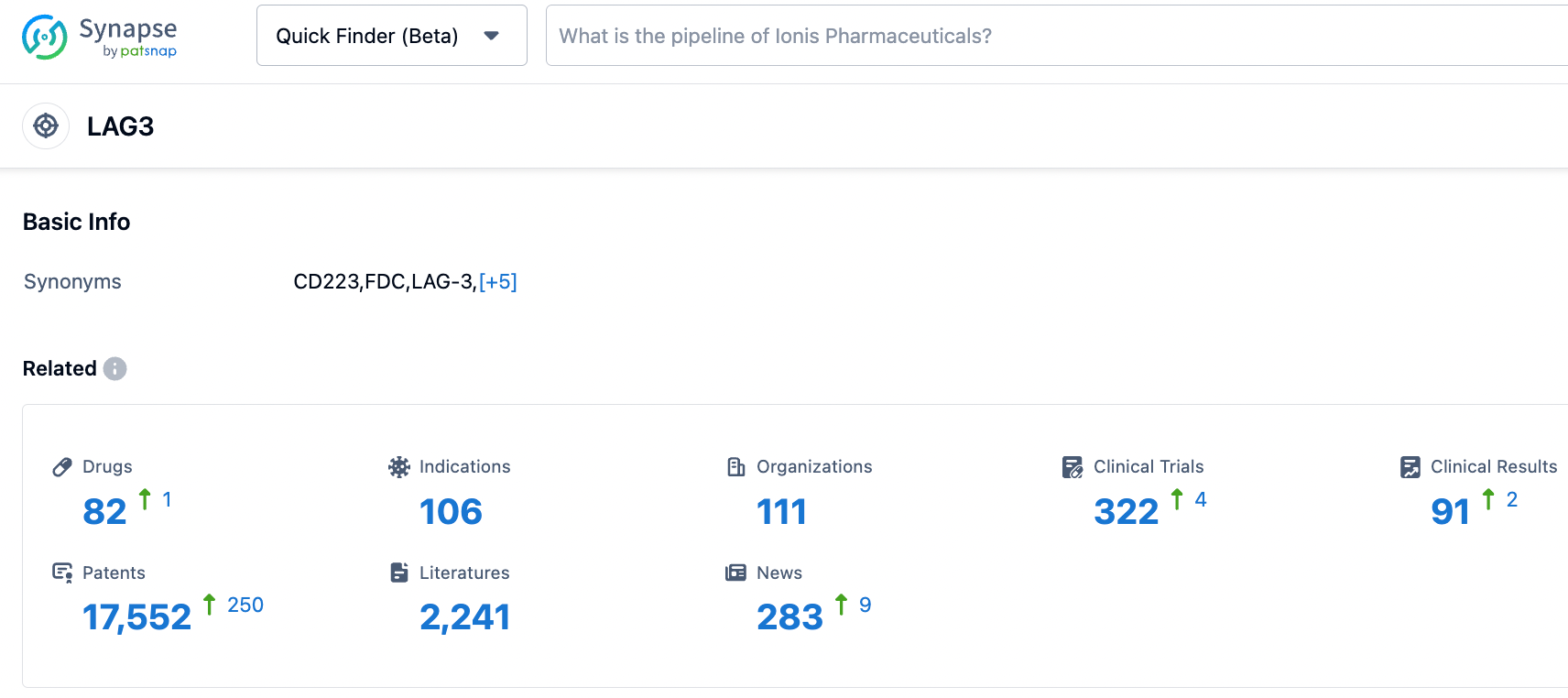
According to information disclosed by the Synapse database as of October 24, 2023, there are 82 drugs under investigation targeting LAG3, covering 106 indications, with 111 institutions involved in research, related to 323 clinical trials, and an extensive 17,549 patents. Efti is a soluble LAG-3 fusion protein, a potential "first-in-class" antigen-presenting cell stimulant. In October 2022, the U.S. FDA granted Efti Fast Track designation, in combination with the anti-PD-1 antibody Keytruda, as a first-line treatment for non-small cell lung cancer (NSCLC). We look forward to the successful development of Efti.