Response Pharmaceuticals Initiates Phase 2 Trial Enrollment for New iMTP Inhibitor RDX-002 in Patients Discontinuing GLP-1 Agonists
Response Pharmaceuticals, Inc., a clinical-stage firm concentrating on weight management and metabolic health for high-risk groups, has officially begun enrollment for a Phase 2 trial to investigate the effects of its drug candidate, RDX-002, on weight rebound following GLP-1 treatment. This study aims to examine how RDX-002, a novel inhibitor of intestinal microsomal triglyceride transfer protein (iMTP), influences postprandial triglyceride levels, as well as its impact on weight and various cardiometabolic risk factors in patients who are stopping the use of GLP-1 agonists, such as semaglutide or tirzepatide, in their obesity treatment regimen.
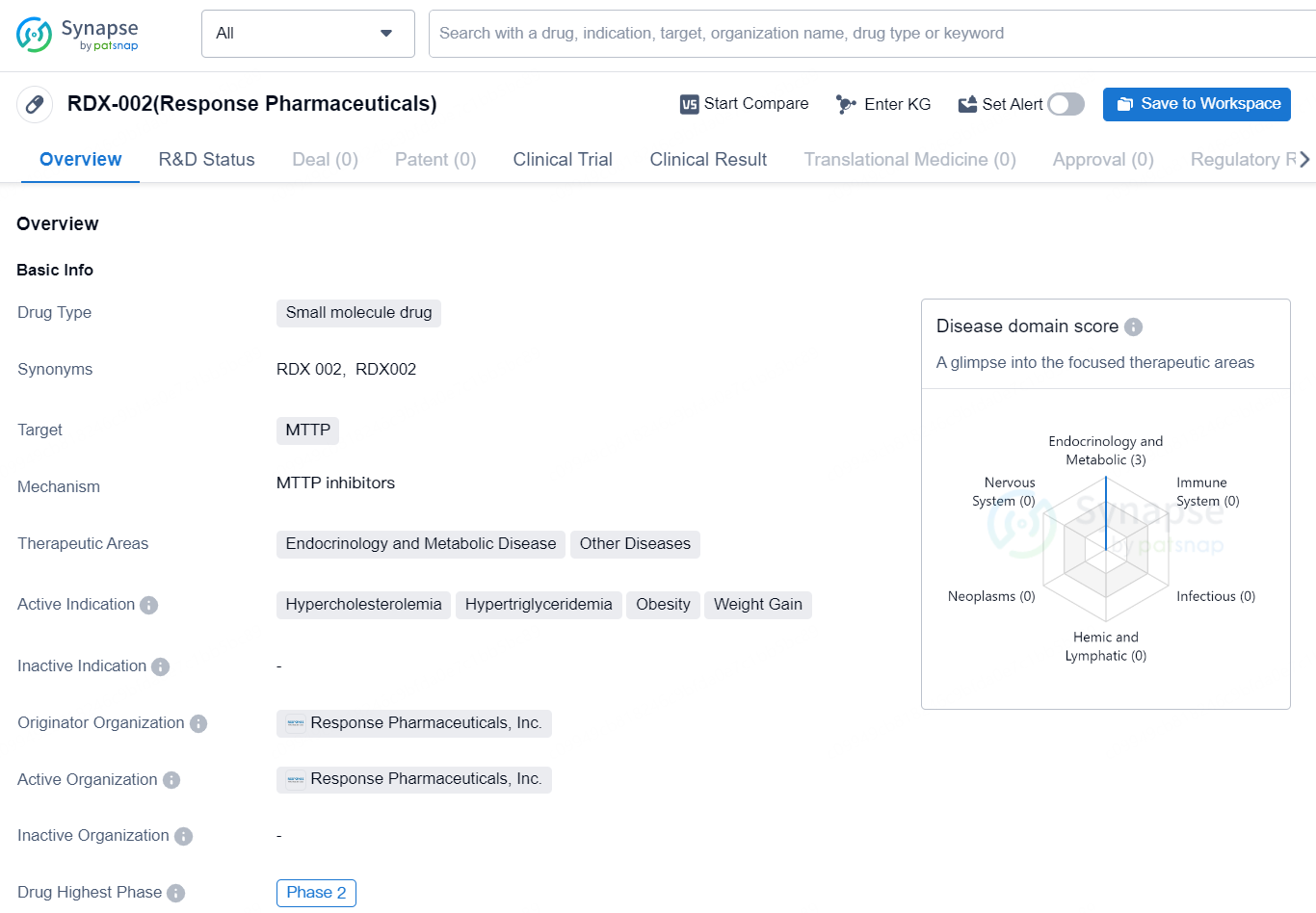
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“There exists a considerable and increasing unmet need for patients who discontinue GLP-1 agonist therapy, as they tend to regain much of the weight lost while undergoing treatment, thereby negating the cardiometabolic advantages gained during this period,” stated Eric Keller, Founder and CEO of Response Pharmaceuticals. “Our early investigations regarding antipsychotic-induced weight gain (AIWG) have indicated that RDX-002 may effectively mitigate weight gain linked to common antipsychotic medications, displaying favorable initial tolerability. We are eager to further assess its impact in this crucial weight management scenario while progressing our development for AIWG.”
Professor Chris Packard from the Institute of Cardiovascular and Medical Sciences at the University of Glasgow commented, “A growing concern in obesity management is the resurgence of body weight and associated cardiometabolic risks following the cessation of GLP-1 agonist therapy. A treatment like RDX-002 could potentially alleviate this weight regain and create a smoother transition for patients after stopping GLP-1 agonists.”
Dr. Bill Sasiela, Chief Medical Officer, pointed out, “Obesity is a chronic, multifaceted condition that heightens the risk for serious cardiovascular and metabolic disorders such as hyperlipidemia, insulin resistance, type 2 diabetes, hypertension, and heart disease. Although GLP-1 agonists such as semaglutide and tirzepatide have proven effective in substantially reducing weight and related cardiovascular/metabolic risks in individuals with obesity, the majority of patients ultimately cease using these medications. RDX-002 shows promise in combating drug-induced weight gain by lowering the absorption of dietary fats in the intestines, which in turn lessens calorie intake. Consequently, this leads to improved outcomes in postprandial triglycerides, “bad” LDL cholesterol, and glycemic control—all recognized risk factors for the development of cardiovascular disease and diabetes.”
The trial (NCT06640972) is a double-blind study examining the effectiveness and tolerability of RDX-002 over a span of 12 weeks in patients who have experienced significant weight loss using GLP-1 agonists and are contemplating stopping the treatment. Results from this study are anticipated in the latter part of 2025.
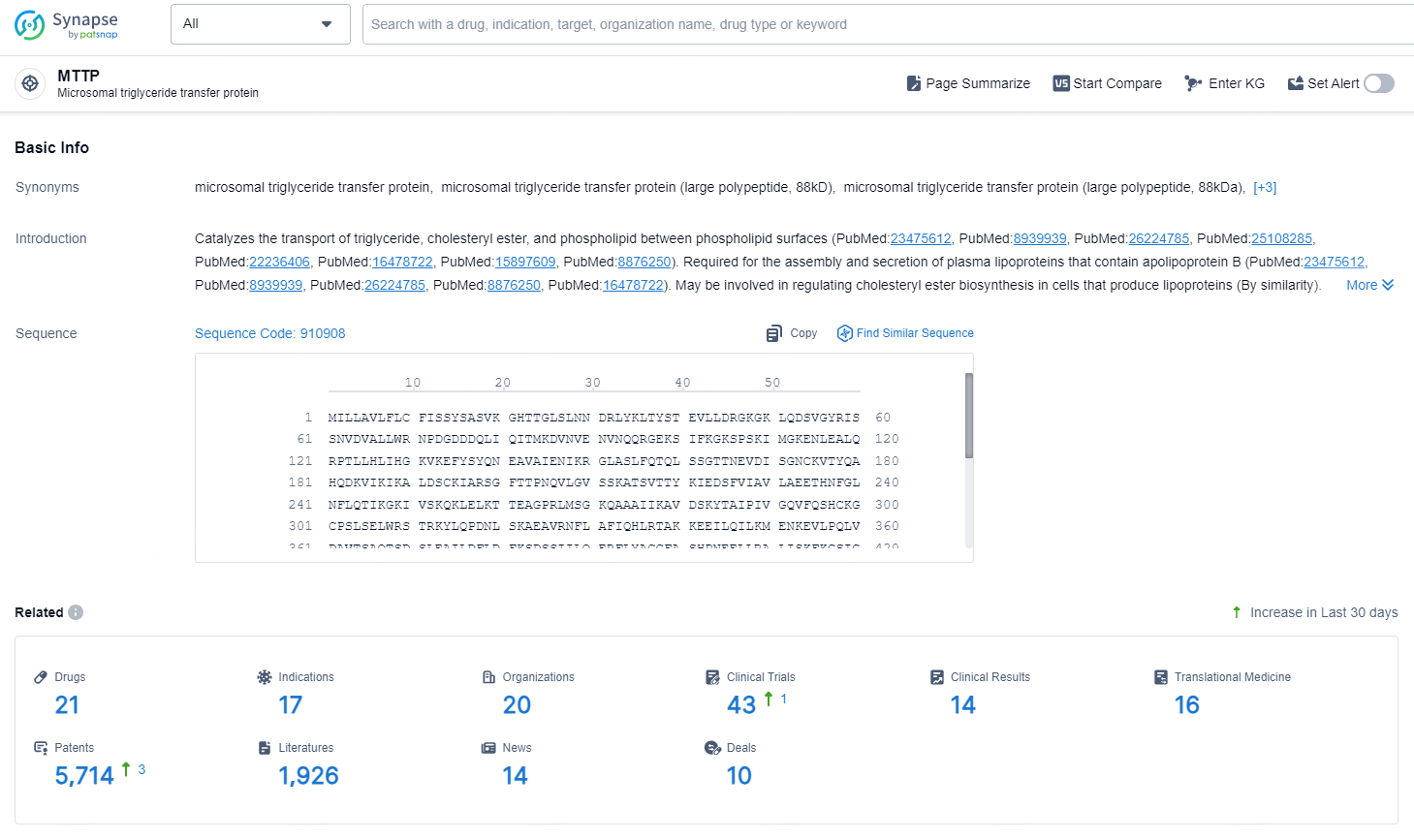
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 21, 2024, there are 21 investigational drug for the MTTP targets, including 17 indications, 20 R&D institutions involved, with related clinical trials reaching 43, and as many as 5714 patents.
RDX-002 (Response Pharmaceuticals) is a small molecule drug developed by Response Pharmaceuticals, Inc. The drug targets MTTP and is being developed for therapeutic areas including Endocrinology and Metabolic Disease, as well as other diseases. The drug is currently in the highest phase globally, Phase 2, indicating that it has shown potential efficacy and safety in early human testing.