Bristol Myers Squibb Reports KRYSTAL-12 Study Success with KRAZATI (Adagrasib) Halting Advanced KRASG12C-Mutant NSCLC Progression
Bristol Myers Squib has declared the success of their critical Phase 3 KRYSTAL-12 trial. In this study, they assessed the efficacy of KRAZATI® (adagrasib) when used as a single-agent treatment for individuals with previously treated local or widespread metastatic non-small cell lung cancer that contains the KRAS G12C mutation. The trial reached its main goal, which was to extend the duration of progression-free survival, and also achieved a significant secondary goal by demonstrating an improvement in the overall response rate. These results were confirmed by an independent review at the final analysis for these respective endpoints.
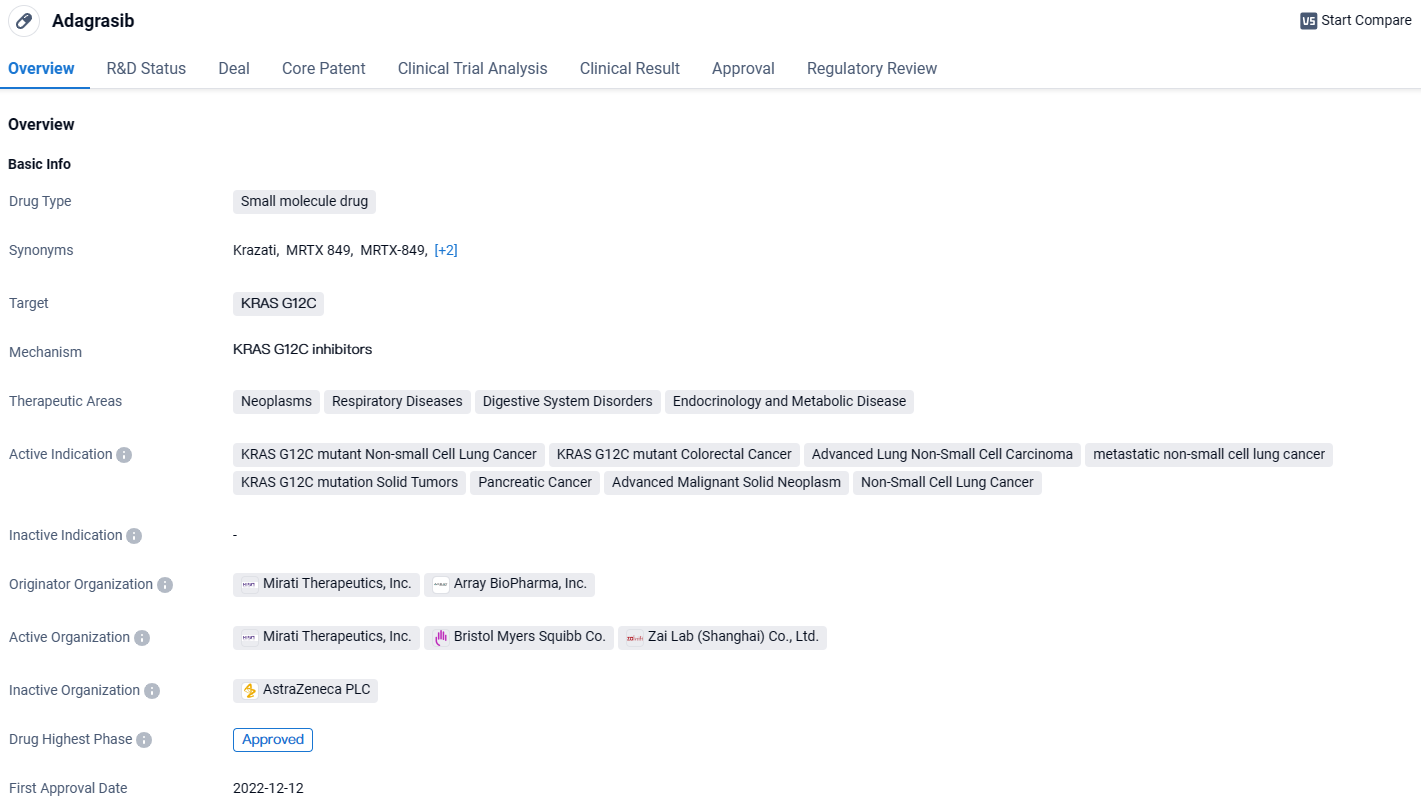
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Research is actively continuing to evaluate an important additional secondary endpoint: the measure of overall survival rates. The confirmatory study's outcomes have indicated that KRAZATI provided a substantial and significant improvement in both Progression-Free Survival (PFS) and Objective Response Rate (ORR), when contrasted with the conventional chemotherapy treatments administered to patients as a subsequent line of therapy. No unfamiliar safety concerns were identified with the use of KRAZATI, and its safety profile aligned with previously established data.
"The recent developments underscore the effectiveness of precision medicine in treating individuals with KRASG12C mutation-positive advanced stage or metastasized lung cancer. The U.S. sanction of KRAZATI has opened up a novel therapeutic path for this patient group, and the initial findings from the KRYSTAL-12 study are anticipated to fortify trust amongst healthcare providers and those affected by the disease," quoted Abderrahim Oukessou, M.D., who is the vice president and global program lead for KRAZATI at Bristol Myers Squibb.
Intent on conducting a thorough analysis of the collected data, the company is eager to present their findings to the scientific circles at a forthcoming clinical meeting and engage with regulatory bodies concerning the insights gathered.
The U.S. Food and Drug Administration previously endorsed KRAZATI for expedited use as a precise therapy for individuals with the KRASG12C mutation in cases of advanced or metastatic Non-Small Cell Lung Cancer (NSCLC) who have tried at least one systemic therapy, back in December 2022.
In 2023, the Medicines and Healthcare products Regulatory Agency issued a conditional marketing approval for KRAZATI as a specific therapy choice for adult individuals living with advanced NSCLC characterized by the KRASG12C mutation, and who have encountered disease progression post-initial systemic treatment. Later, the European Commission followed suit with its authorization in 2024.
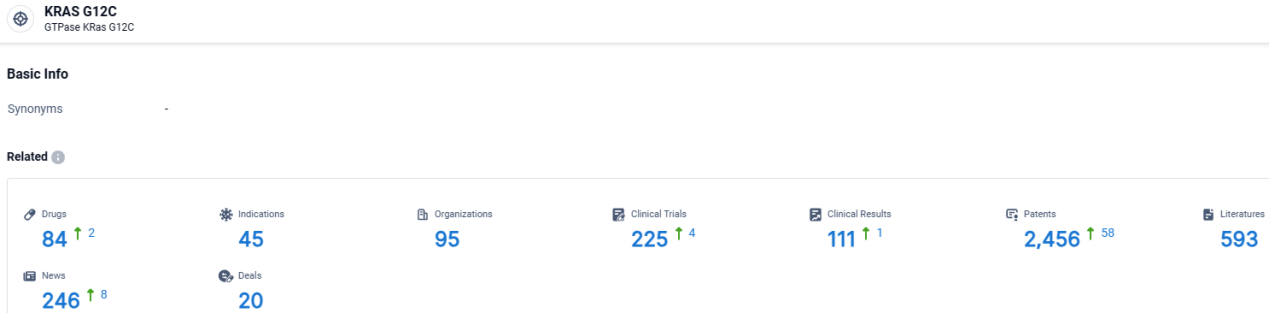
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 2, 2024, there are 84 investigational drugs for the KRAS G12C target, including 45 indications, 95 R&D institutions involved, with related clinical trials reaching 225, and as many as 2456 patents.
Adagrasib is a promising drug in the field of biomedicine, specifically targeting the KRAS G12C mutation. Its approval in the United States and ongoing phase 3 development in China indicate its potential effectiveness in treating various cancers and other related diseases. The drug's multiple regulatory designations further highlight its significance and potential impact in the pharmaceutical industry.