Bristol Myers Squibb Invests $1.4 Billion in Layout for Schizophrenia Treatment Candidate Drugs
On March 18th, Bristol Myers Squibb (BMS) announced that it had completed the acquisition of Karuna Therapeutics, a move that will further strengthen its investment portfolio in the field of neuroscience. With the acquisition finalized, Karuna Therapeutics has ceased trading on the NASDAQ Global Select Market and is now a wholly-owned subsidiary of Bristol Myers Squibb.
At the end of December last year, BMS and Karuna jointly announced that the parties had reached a definitive merger agreement. According to the terms of the agreement, Bristol Myers Squibb will acquire all outstanding shares of Karuna at a cash price of $330.00 per share, with a total equity value of approximately $14 billion. After deducting the estimated cash acquired, the net transaction value is approximately $12.7 billion.
Karuna is a biopharmaceutical company focused on the discovery, development, and delivery of innovative treatments that change the lives of patients with psychiatric and neurological disorders. Its lead product in development, KarXT (xanomeline-trospium), is an antipsychotic drug with a novel mechanism of action that offers unique efficacy and safety. The New Drug Application (NDA) for KarXT for the treatment of adult schizophrenia submitted by Karuna has been accepted for review by the U.S. FDA, with a Prescription Drug User Fee Act (PDUFA) target action date set for September 26, 2024. KarXT is also undergoing registrational trials exploring its potential as an adjunctive therapy to existing standard-of-care medications for schizophrenia and for the treatment of psychosis in Alzheimer's disease patients.
Regarding Schizophrenia
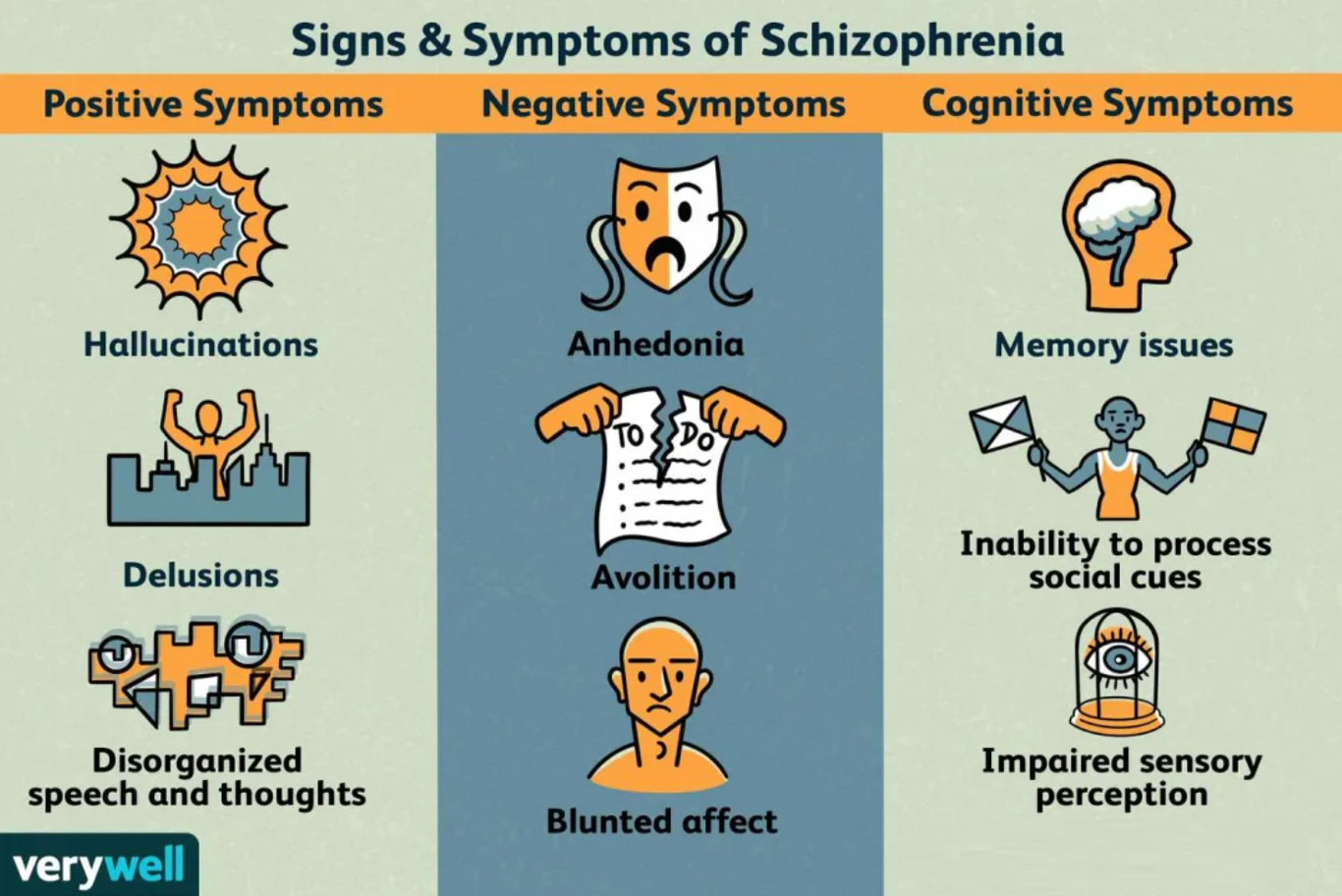
Schizophrenia is a chronic, severe, and debilitating mental disorder. When schizophrenia is active, symptoms can include delusions, hallucinations, disorganized speech, thought disorders, and a lack of motivation. There are 20 million people worldwide who suffer from schizophrenia. With treatment, most symptoms of schizophrenia can greatly improve, and the possibility of relapse can be reduced.
The impact of schizophrenia is equivalent in males and females, but males may develop the disease earlier. The prevalence rate is similar worldwide. Compared to the general population, people with schizophrenia are more likely to die prematurely, mainly due to a higher prevalence of diseases such as heart disease and diabetes.
The complexity of schizophrenia may help explain why there are misconceptions about the disorder. Schizophrenia does not mean a split personality or multiple personalities. Most people with schizophrenia are not more dangerous or violent than the general public. Although limited mental health resources in the community can lead to homelessness and frequent hospitalizations for individuals with schizophrenia, the belief that individuals with schizophrenia end up homeless or in hospitals is a misconception.
Karuna Therapeutics
Karuna Therapeutics was established in Boston, the mecca of global biotechnology companies. Its parent company Puretech Health is revered as a respected industry leader within the Boston biotech community, founded by Daphne Zohar, who is also the founder and CEO of Puretech Health.
Ms. Daphne Zohar, founder of Puretech Health, has been recognized as one of the top innovators under 35 by MIT Technology Review. She was chosen as one of the 28 top female leaders globally by BioWorld, named one of the "Top 15 Innovators" in the Boston area by The Boston Globe, recognized as a woman to watch by the Boston Business Journal, and MedCity News acknowledged her as one of the most influential women in healthcare innovation and on Twitter.
Another founding figure of Puretech Health is Robert Langer, the company's key strategist working behind the scenes. He is a distinguished professor at MIT and a member of all three national academies. He has published more than 1,400 research papers and holds over 1,300 issued and pending patents worldwide. His patents have been licensed or sublicensed to more than 350 pharmaceutical, chemical, biotechnology, and medical device companies. He is a world-renowned expert in the field of biomedical engineering. Robert Langer has been successful in transforming scientific discoveries and research findings in the laboratory into real-world therapeutic methods, diagnostic reagents, devices, and medicines.
According to the official website of PureTech Health, the company's vision is "Giving Life to Science." PureTech Health transforms scientific and technological advancements into practical clinical applications by creating companies.
PureTech Health has incubated several subsidiaries, aiming to develop new drugs in various different directions. Among them, Karuna's mission is to advance transformative medications for patients with psychiatric and neurological disorders.
In May 2012, Karuna signed an exclusive licensing agreement with Eli Lilly. Under the terms of this agreement, Eli Lilly transferred to Karuna all rights related to certain patents, regulatory documents, data records, and materials associated with Xanomeline. Karuna obtained the authority to sublicense or otherwise transfer rights related to the licensing agreement with Eli Lilly and is obliged to make commercially reasonable efforts to develop, produce, and commercialize any dosage form of Xanomeline, as well as to seek and maintain regulatory approval for human use.
This collaboration resulted in the most significant R&D pipeline for Karuna: KarXT (Karuna-Xanomeline-Trospium), intended for the treatment of psychosis-related symptoms in patients with schizophrenia and Alzheimer's disease.
Looking back at 2018, it was a pivotal year for Karuna Therapeutics.
In mid-June, Karuna received an $8 million Wellcome Trust Award grant from the Wellcome Trust to advance its key pipeline, KarXT (Karuna-Xanomeline-Trospium), in Phase 2 clinical trials for the treatment of schizophrenia. This represented additional funding following the initial $3.8 million awarded by the Wellcome Trust to Karuna for Phase 1 clinical research of KarXT (Karuna-Xanomeline-Trospium).
KarXT (Karuna-Xanomeline-Trospium) is a combination formulation comprising Trospium Chloride and Xanomeline, which works as an agonist of the muscarinic M1 and M4 receptors and as an antagonist of peripheral muscarinic receptors with restrictive properties. Karuna initiated a Phase 1 clinical study of KarXT for the treatment of schizophrenia in 2016, which was completed in mid-2017.
In August, Karuna announced the completion of its Series A financing round, raising $42 million, led by ARCH Venture Partners. Among the investors in this round was an individual investor, the founder of publicly traded company SAGE Therapeutics, Steven Paul. Subsequently, Karuna announced the appointment of Steven Paul as the company's new CEO.
Steven Paul possesses extensive experience in the research and development of central nervous system (CNS) drugs. He worked at Eli Lilly for 17 years, where he held several key leadership roles, including the President of Lilly Research Laboratories. At Eli Lilly, he was responsible for the company's overall R&D efforts and helped oversee the development of CNS drugs such as Zyprexa® and Cymbalta®. Most critically, he led the clinical development research of the xanomeline project at Eli Lilly for the treatment of Alzheimer's disease and obtained clinical evidence of xanomeline's antipsychotic and pro-cognitive properties.
Between 2018 and 2022, Steven Paul led the rapid growth and transformation of Karuna.
Karuna's Drug Pipeline
According to the latest corporate presentation by Karuna, KarXT is the company's most important research and development pipeline.
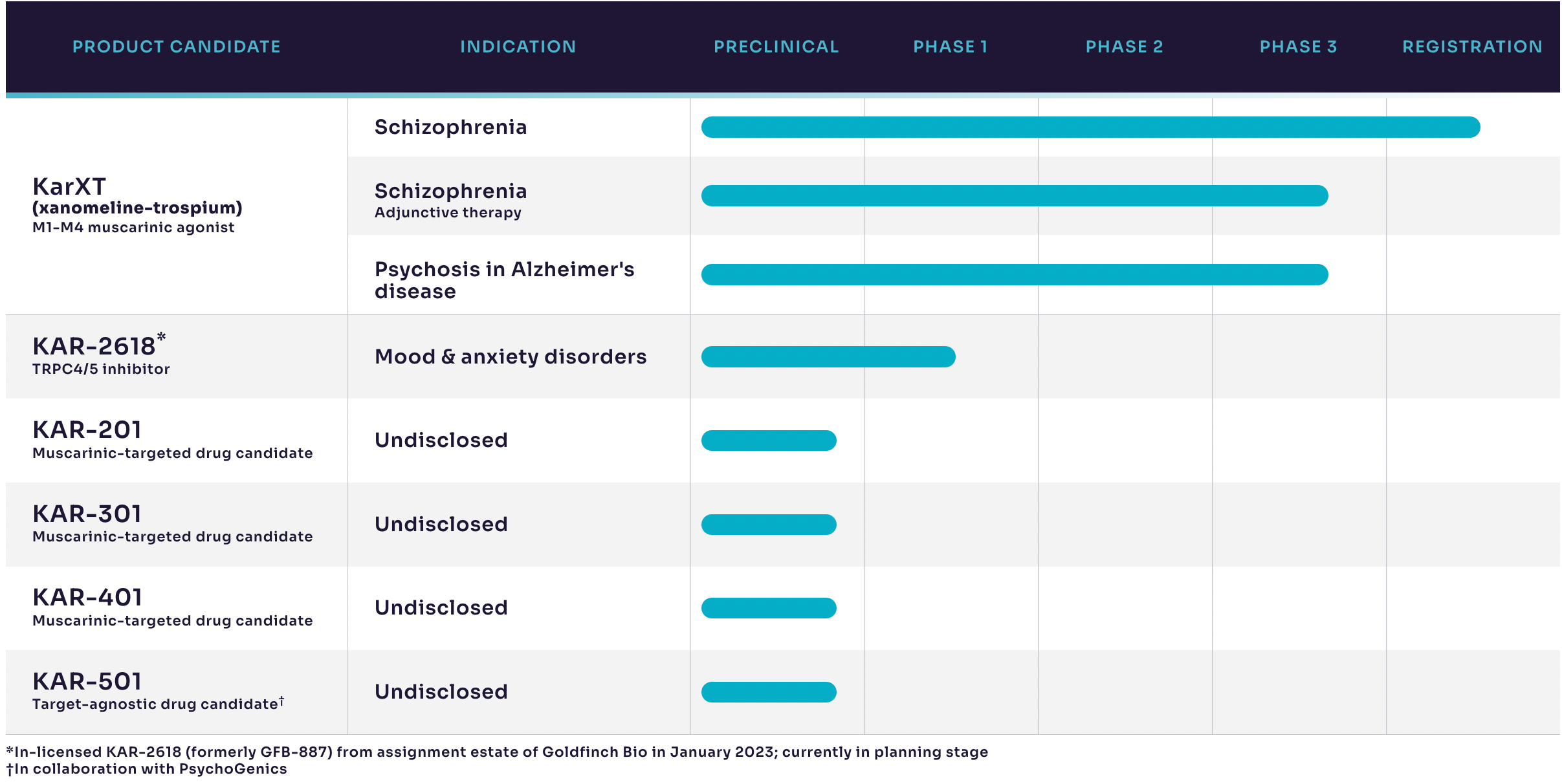
In three 5-week clinical studies (EMERGENT-1/2/3), patients with schizophrenia experienced clinically meaningful improvements in symptoms. Data on patients' positive symptoms, negative symptoms, and secondary endpoints were all highly significant.
The company has submitted a marketing application in the third quarter of 2023 and will continue to complete multiple long-term studies on the safety and tolerability over the next two years. Clinical trials for KarXT in the treatment of Alzheimer’s disease are also set to commence.
Additionally, in February 2023, the company entered into a global exclusive licensing agreement with Goldfinch Bio for the research of TRPC4/5 candidate products. Under this agreement, Karuna will obtain the global exclusive rights to develop, manufacture, and commercialize multiple TRPC4/5 candidates, including the lead clinical-stage candidate GFB-887. Goldfinch Bio will receive an upfront payment of $15 million and up to $520 million in potential milestone payments, as well as royalties on each TRPC4/5 candidate product.
Karuna will share details of the development plan for GFB-887 in the treatment of mood and anxiety disorders in the second half of 2023.
Other Representative Schizophrenia Candidate Drug
Brilaroxazine is a novel multimodal partial agonist for 5-HT1A, 5-HT2A, D2, D3, D4 receptors, and an antagonist for 5-HT2B, 5-HT2C, 5-HT7R, developed by Reviva Pharmaceuticals.
In 2017, Reviva Pharmaceuticals published preclinical study results about Brilaroxazine as an atypical antipsychotic, indicating that Brilaroxazine could improve declarative memory and psychotic symptoms in a mouse model of schizophrenia. Furthermore, the drug also has the potential to treat idiopathic pulmonary fibrosis and pulmonary arterial hypertension.

On October 30th, Reviva Pharmaceuticals announced that the global pivotal Phase 3 RECOVER trial for its investigational drug Brilaroxazine achieved positive preliminary results. Compared to placebo, a 50 mg dose of Brilaroxazine resulted in a reduction of 10.1 points in the PANSS total score (a scale that measures positive and negative syndromes of schizophrenia) at week 4, with significant statistical and clinical relevance.
In terms of safety, after 4 weeks of treatment, no drug-related serious adverse events (SAEs) were observed, no treatment-emergent SAEs (TESAEs) were seen, and no significant safety concerns for Brilaroxazine were reported.
Roluperidone (MIN-101) is a clinical-stage small molecule drug developed by Minerva Neurosciences that acts as a dual antagonist of the 5-HT2A receptor and sigma2 receptor.
On May 10th, Minerva Neurosciences announced that the New Drug Application for Roluperidone for the treatment of negative symptoms in patients with schizophrenia has been submitted for FDA review.

Ulotaront is a dual agonist of the 5-HT1A receptor and Trace Amine-Associated Receptor 1 (TAAR1), originally developed by Sumitomo Pharma, and is currently being investigated as an adjunctive therapy for the treatment of schizophrenia, generalized anxiety disorder, and major depressive disorder.
On July 31st, Sumitomo Pharma announced on their official website that their antipsychotic clinical candidate drug, Ulotaront (SEP-363856), did not meet the primary endpoints in two recent Phase 3 clinical studies (DIAMOND 1 and DIAMOND 2).




