Centessa Pharmaceuticals Presents Key Data on ORX142, a Novel OX2R Agonist, from Non-Human Primate Study
Centessa Pharmaceuticals plc (Nasdaq: CNTA) revealed that preclinical findings from an NHP (non-human primate) study of ORX142—a potent and highly selective agonist of the orexin receptor 2 (OX2R) aimed at treating excessive daytime sleepiness (EDS) associated with certain neurological, neurodegenerative, and psychiatric conditions—will be presented as a late-breaking poster at the 27th Congress of the European Sleep Research Society (Sleep Europe 2024), scheduled for September 24-27, 2024, in Seville, Spain.
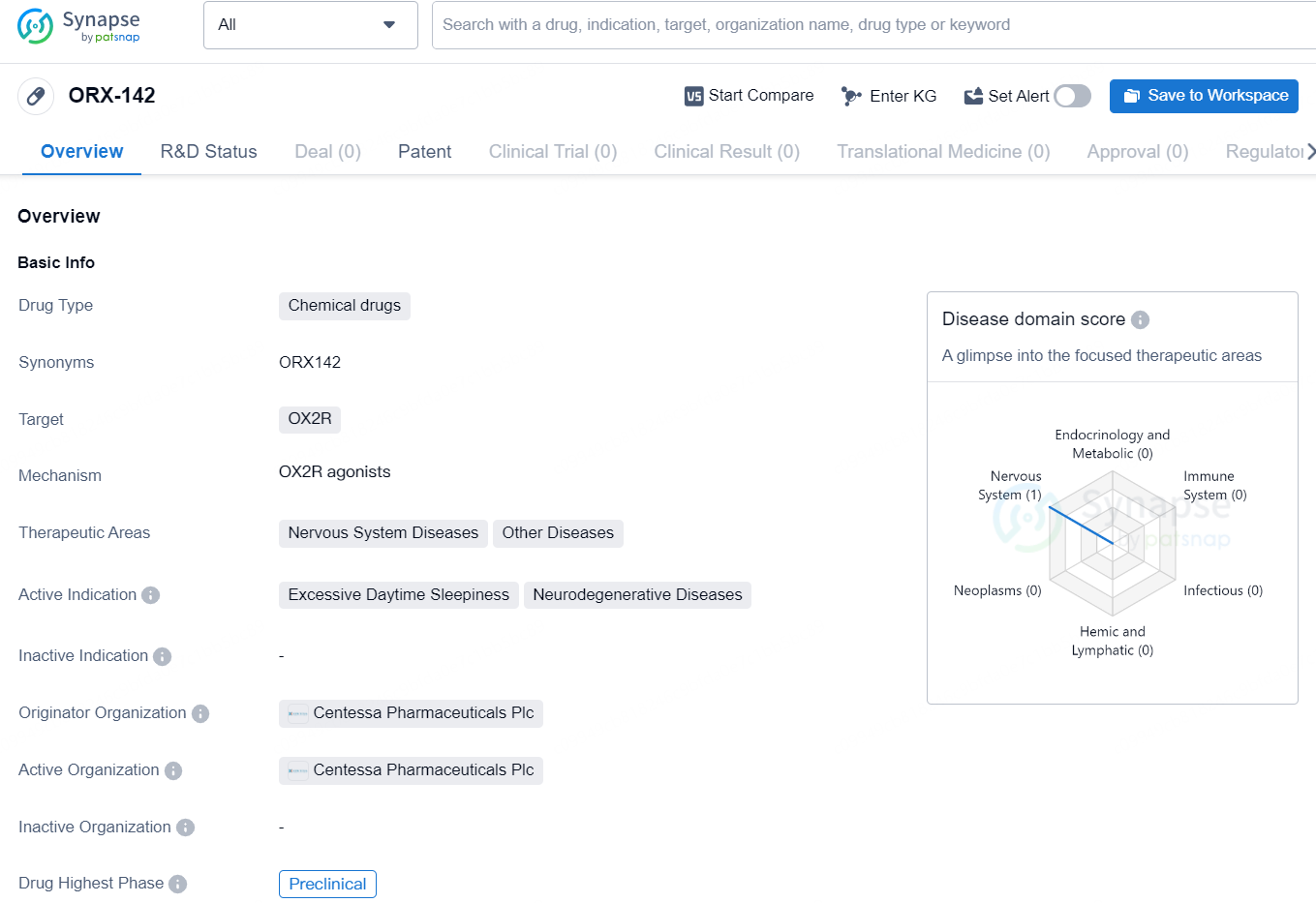
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The poster presentation will showcase, for the first time, robust preclinical findings indicating that low doses of ORX142 enhanced wakefulness in non-human primates using a highly predictive and translational model.
"ORX142 represents the second drug candidate from our expanding portfolio of potentially best-in-class OX2R agonists that has demonstrated significant efficacy in promoting wakefulness at very low doses in highly predictive and translational preclinical models," stated Saurabh Saha MD PhD, Chief Executive Officer of Centessa. "We believe these non-human primate findings are persuasive as they highlight the potential for ORX142, a highly potent and novel OX2R agonist, to combat excessive daytime sleepiness (EDS) in various neurological, neurodegenerative, and psychiatric disorders without significant orexin loss. We are excited to present these preclinical data in a late-breaking session at Sleep Europe 2024."
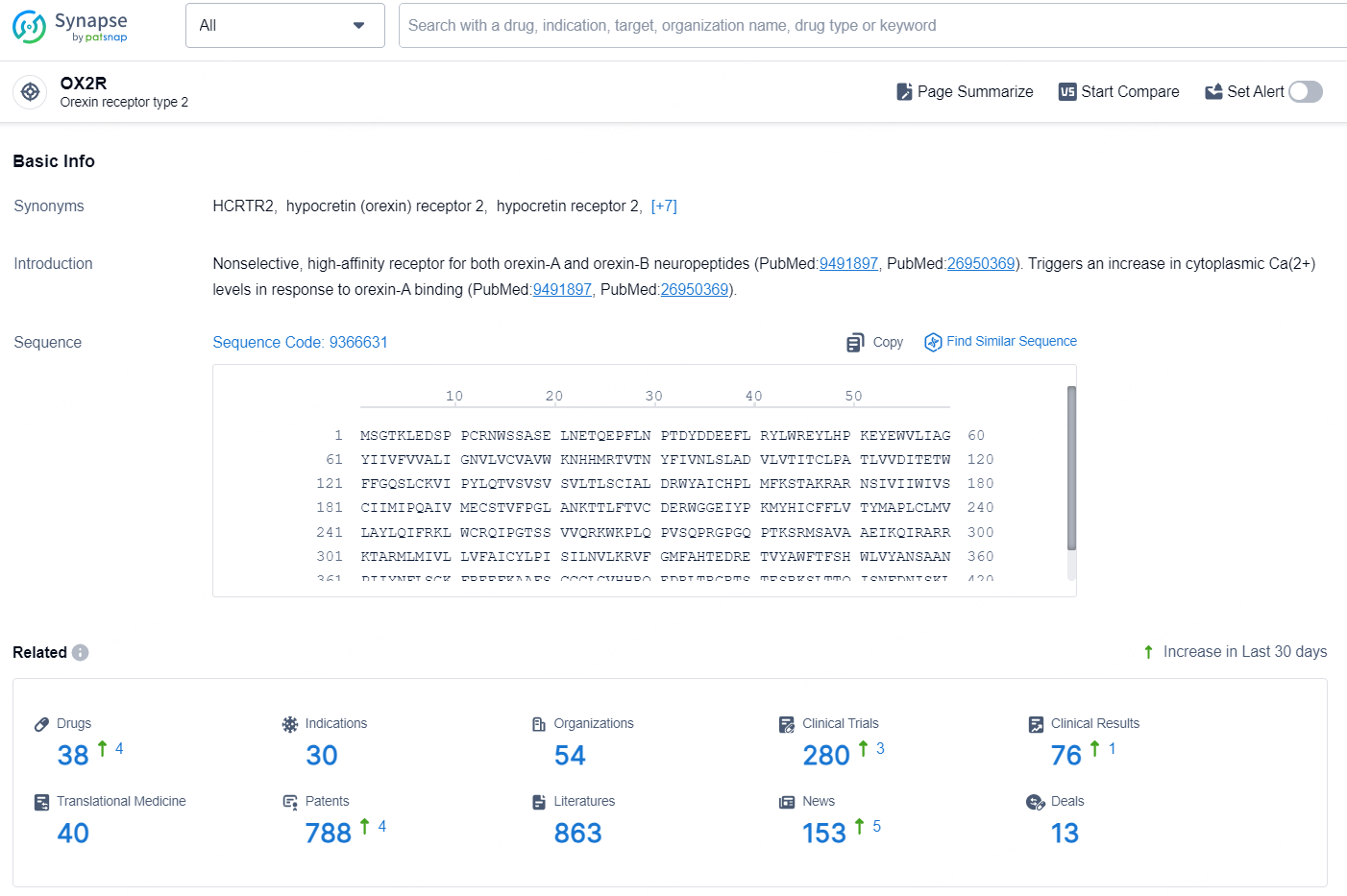
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 30, 2024, there are 38 investigational drugs for the OX2R targets, including 30 indications, 54 R&D institutions involved, with related clinical trials reaching 280, and as many as 788 patents.
The drug ORX-142 is classified as a chemical drug and targets the OX2R receptor. It is intended for the treatment of nervous system diseases and other diseases, with a focus on addressing excessive daytime sleepiness and neurodegenerative diseases. ORX-142 is currently in the preclinical phase, indicating that it has not yet progressed to clinical trials. The drug is developed by Centessa Pharmaceuticals Plc, a pharmaceutical organization that specializes in the development of innovative therapies for various medical conditions.