Cerevance doses first participant for CVN293 in the Phase 1 Clinical Trial, to potentially treat ALS and Alzheimer's Disease
Cerevance, a corporation engaged in clinical-stage drug discovery, dedicated to pushing forward new and accurate neuroscience treatments for CNS ailments by utilizing their exclusive Nuclear Enriched Transcript Sort sequencing technology, has reported the initiation of their Phase 1 trial with the first participant receiving a dose for the purpose of assessing the safety, tolerability, and pharmacokinetics of CVN293.
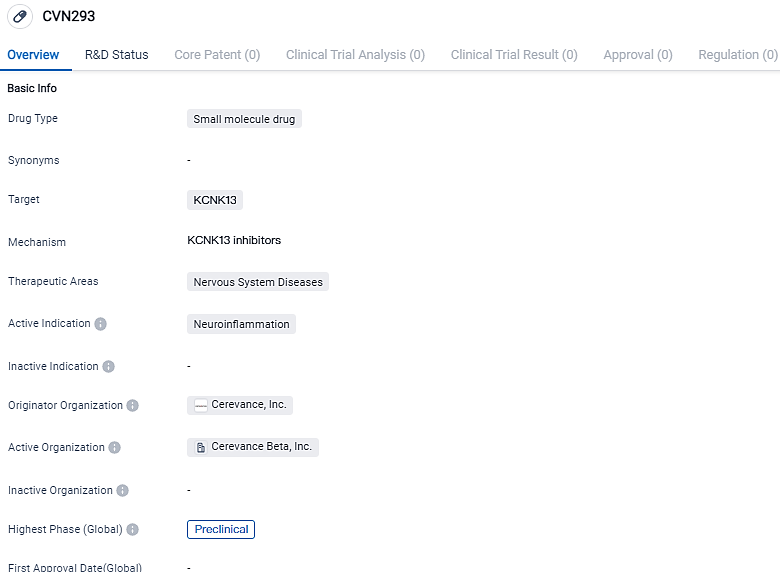
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Cerevance is thrilled to move forward with CVN293, our proprietary small molecule that inhibits the unique target KCNK13, into its first phase of clinical trials," stated Craig Thompson, the company's CEO.
"Identified specifically through NETSseq, KCNK13 is a crucial player in neuroinflammation, illustrating the potency of Cerevance's platform for discovering targeted neuroscience applications. It is a significant step towards transforming scientific breakthroughs into potential therapy options for neuroinflammatory disorders," Thompson further added.
Our initial clinical trial of phase 1 will combined single ascending dose and multiple ascending dose study that aims to examine the safety, tolerability, and pharmacokinetics of CVN293 when orally given to healthy test subjects. Single or repeated dosages of CVN293 will be administered in a randomized, placebo-controlled, double-blinded, dose-escalation setup.
We anticipate enlisting 64 healthy male and female participants ranging from 18 to 55 years of age. The SAD study will include 5 groups of 8 participants each, while the MAD study will contain 3 groups of 8 participants. Each group will be randomized at a 6:2 ratio to get either CVN293 or a placebo.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
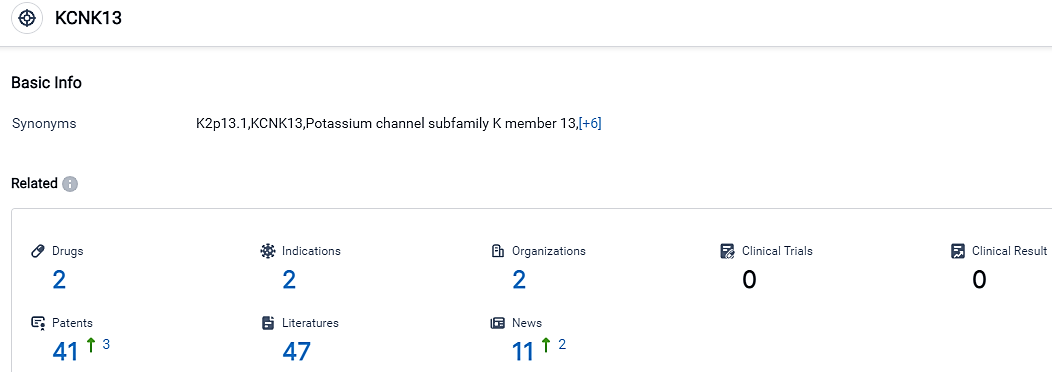
According to the data provided by the Synapse Database, As of September 25, 2023, there are 2 investigational drugs for the KCNK13 target, including 2 indications,2 R&D institutions involved, and as many as 41 patents.
CVN293 is an investigational, potent, and selective inhibitor of KCNK13, a novel target shown by NETSseq to be selectively expressed in brain microglia, a cell type that is central in neuroinflammation and has been implicated in many neurodegenerative diseases. The potential benefits of CVN293's selective action could mean a more targeted, precision neuroscience approach to treating neuroinflammation.