Compugen Announces FDA Clearance of IND for COM503 for the Treatment of Solid Tumors
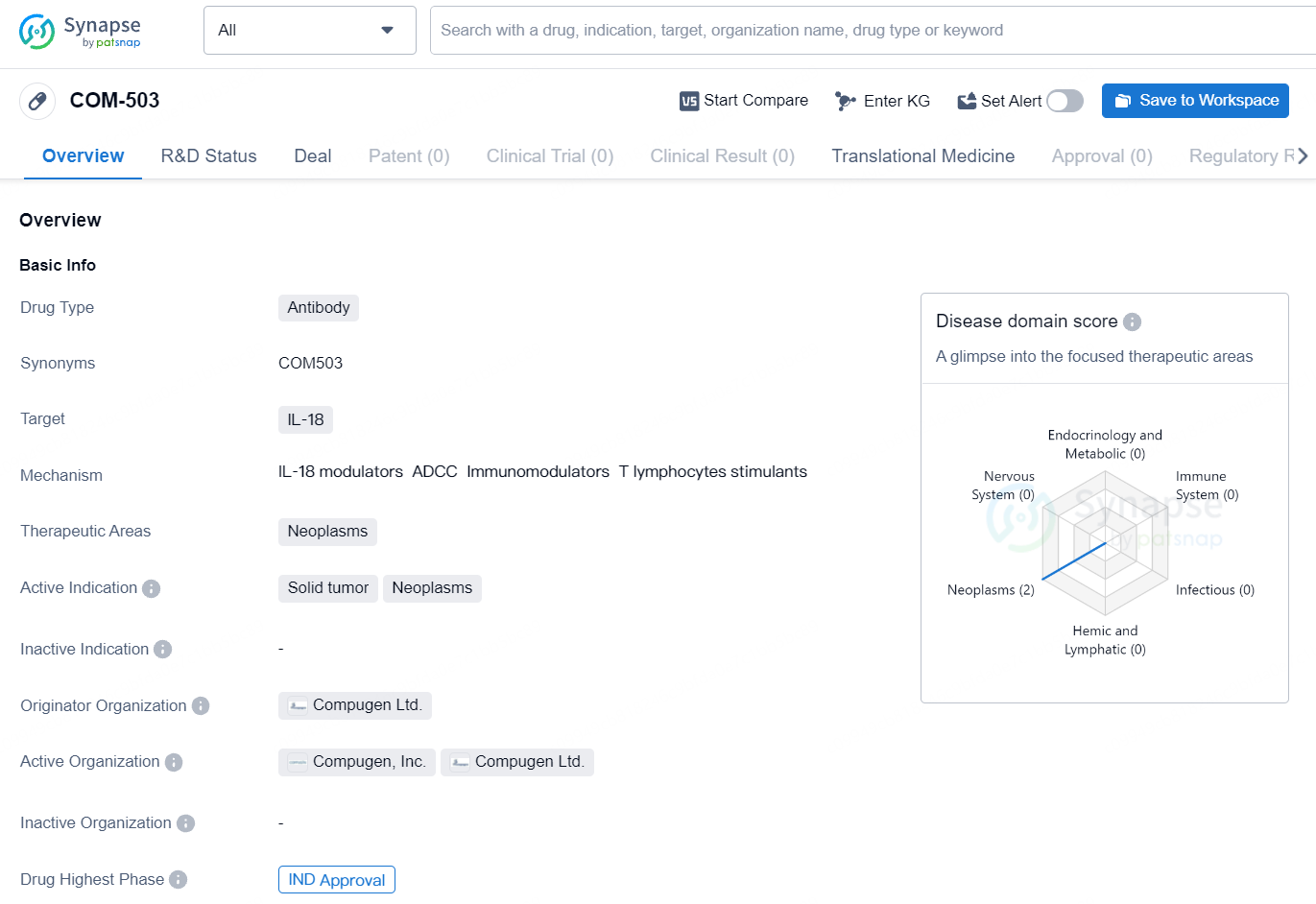
Compugen Ltd., a company in the clinical-stage of cancer immunotherapy and a leader in computational target discovery, has announced that the U.S. Food and Drug Administration has approved the investigational new drug application to begin a Phase 1 trial for COM503. This investigational drug is a potential first-in-class, high affinity anti-IL-18 binding protein antibody, licensed to Gilead Sciences, Inc.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 Following IND clearance, Gilead made a $30 million milestone payment to Compugen. The company is set to begin a Phase 1 trial for solid tumors in Q4 2024.
Following IND clearance, Gilead made a $30 million milestone payment to Compugen. The company is set to begin a Phase 1 trial for solid tumors in Q4 2024.
"We are ecstatic to obtain FDA IND clearance for COM503, which activates a $30 million milestone payment from Gilead and allows us to proceed with the Phase 1 trial, accelerating the development of COM503," stated Anat Cohen-Dayag, Ph.D., President, and CEO of Compugen. "We are enthusiastic about COM503's potential. It represents a unique antibody strategy to leverage cytokine biology for cancer treatment, discovered via our computational efforts at Compugen."
Dr. Cohen-Dayag further commented, "This milestone exemplifies our consistent performance and the diversity of our pipeline, marking another clinical program birthed from our predictive computational discovery platform. Additionally, it bolsters our financial standing, giving us enough funds to potentially carry us through 2027. Our preparations for the Phase 1 trial, which is slated to begin in the fourth quarter of this year, are well underway."
The Phase 1 trial will be a first-in-human, dose escalation, and dose expansion study to evaluate the safety and tolerability of COM503 as a standalone therapy and in combination with Gilead's anti-PD-1, zimberelimab, in patients with advanced or metastatic solid tumors worldwide.
In 2023, Compugen and Gilead signed a license agreement, granting Gilead exclusive rights to develop and market anti-IL-18 binding protein antibodies, including COM503. Compugen retains responsibility for preclinical development and the first-in-human Phase 1 trial to assess COM503's safety and tolerability.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
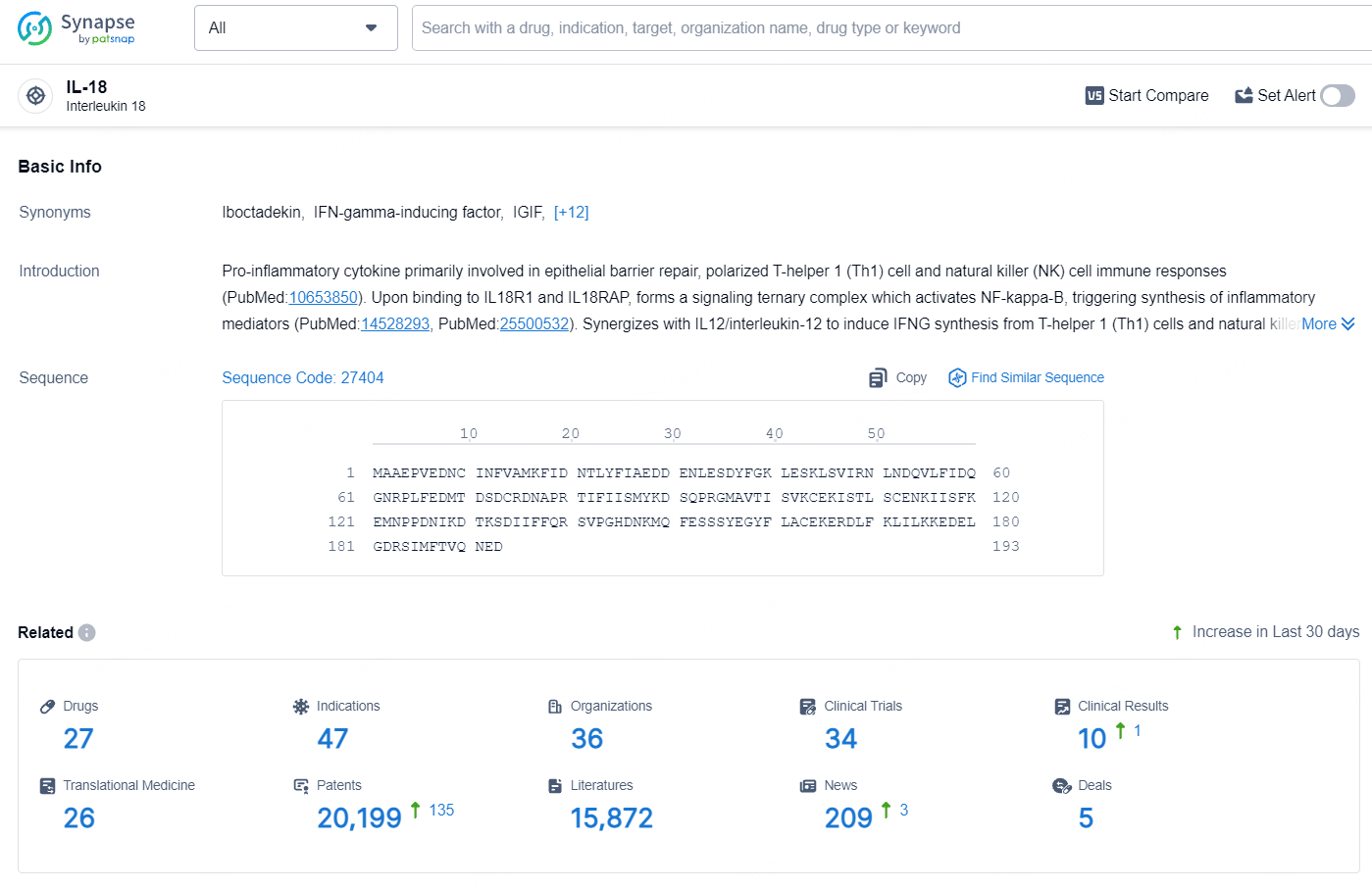
According to the data provided by the Synapse Database, As of August 1, 2024, there are 27 investigational drugs for the IL-18 targets, including 47 indications, 36 R&D institutions involved, with related clinical trials reaching 34, and as many as 20199 patents.
COM-503 has the potential to be a novel therapeutic option for neoplastic diseases, particularly in targeting IL-18. However, as it is still in the preclinical phase, further research and clinical trials will be needed to determine its safety and efficacy in human patients.