Is AREXVY approved by the FDA?
Yes, Arexvy is FDA approved. The FDA approved Arexvy on May 3, 2023. It is the first respiratory syncytial virus (RSV) vaccine approved for older adults, specifically for those aged 60 years and over, to prevent severe RSV symptoms and reduce the risk of hospitalization or death from RSV.
What is Arexvy?
Arexvy is a vaccine designed to prevent severe symptoms of RSV in older adults. RSV is a common, contagious virus that can cause serious respiratory illness, especially in older adults with underlying health conditions such as diabetes, chronic heart, and lung diseases.
Indications and Usage
Arexvy is used to:
- Prevent severe RSV symptoms in adults aged 60 years and over.
- Reduce the risk of hospitalization or death due to RSV.
- It may also be administered to adults aged 50 through 59 years with certain chronic medical conditions that increase their risk of lower respiratory tract disease (LRTD) caused by RSV.
How Does Arexvy Work?
Arexvy helps the body build an immune response by inducing antibodies against the RSVpreF3 antigen, the main protein found on the surface of RSV. This immune response helps prevent severe symptoms of LRTD caused by RSV. Clinical trials showed 94.6% efficacy in reducing the risk of developing RSV-associated LRTD in older adults with underlying conditions, with an overall efficacy of 82.6%.
Dosage and Administration
- Dosing: Arexvy is administered as a single 0.5 mL injection into the muscle of the upper arm (deltoid muscle).
- Preparation: The vaccine is supplied in two vials that must be reconstituted before administration:
- One vial contains lyophilized recombinant RSV glycoprotein F (RSVPreF3) as the antigen component.
- The other vial contains GSK’s proprietary AS01E adjuvant as the adjuvant suspension component.
Storage Instructions
- Before Reconstitution:
- Store refrigerated between 2°C and 8°C (36°F and 46°F).
- Store in the original package to protect vials from light.
- Do not freeze. Discard if frozen.
- After Reconstitution:
- Administer immediately or store in the refrigerator between 2°C and 8°C (36°F to 46°F) or at room temperature (up to 25°C / 77°F) for up to 4 hours.
- Protect from light.
- Discard if not used within 4 hours or if frozen.
Side Effects
Common side effects of Arexvy include:
- Injection site pain (61%)
- Fatigue (34%)
- Muscle aches (29%)
- Headache (27%)
- Joint pain (18%)
Side effects are generally temporary and do not last long.
Warnings and Precautions
- Anaphylaxis: Possible anaphylactic reactions may occur. Do not administer Arexvy to anyone with a known severe allergic reaction to it or any of its components. Ensure administration in a facility capable of managing anaphylactic reactions.
- Syncope (fainting): Fainting may occur post-vaccination. Procedures should be in place to avoid injury.
- Immunosuppression: Immunocompromised individuals or those on immunosuppressive therapy may have a diminished response to the vaccine.
- Pregnancy and Lactation: Arexvy is not approved for use in pregnant or breastfeeding women.
- Age Restrictions: Arexvy is not approved for individuals under 50 years of age or for children.
Ingredients
- Active Ingredients: 120 mcg of the recombinant RSVPreF3 antigen, 25 mcg of MPL, and 25 mcg of QS-21.
- Inactive Ingredients: Trehalose, sodium chloride, potassium dihydrogen phosphate, dipotassium phosphate, polysorbate 80, disodium phosphate anhydrous, DOPC, and cholesterol.
- Preservatives: Arexvy contains no preservatives. The vial stoppers are not made with natural rubber latex.
Conclusion
Arexvy, approved by the FDA on May 3, 2023, is a groundbreaking vaccine for preventing severe RSV symptoms in older adults. By inducing an immune response against RSV, Arexvy helps reduce the risk of hospitalization or death from this common virus, especially in those with underlying health conditions. Always consult with your healthcare provider for personalized medical advice and information about this vaccine.
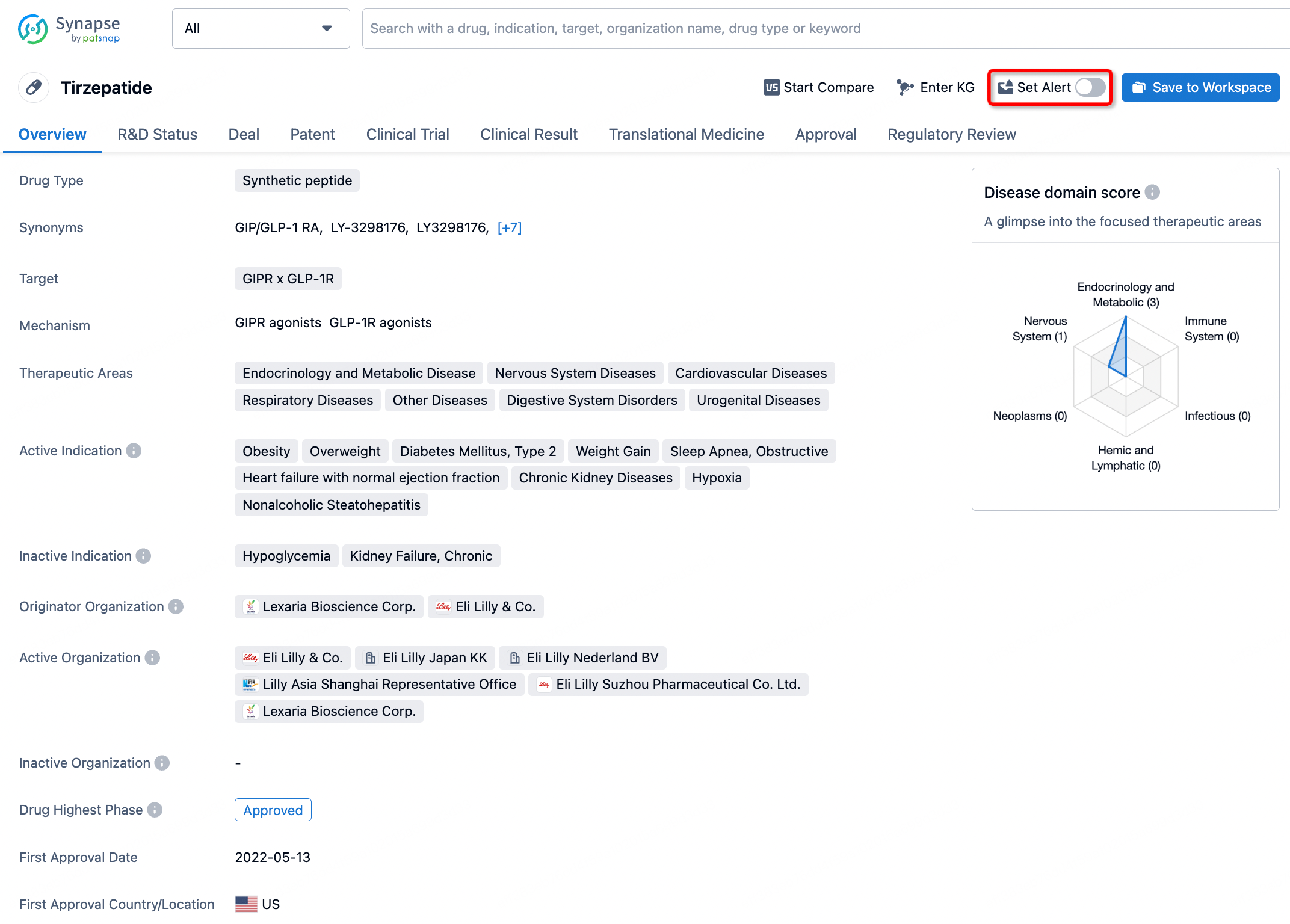
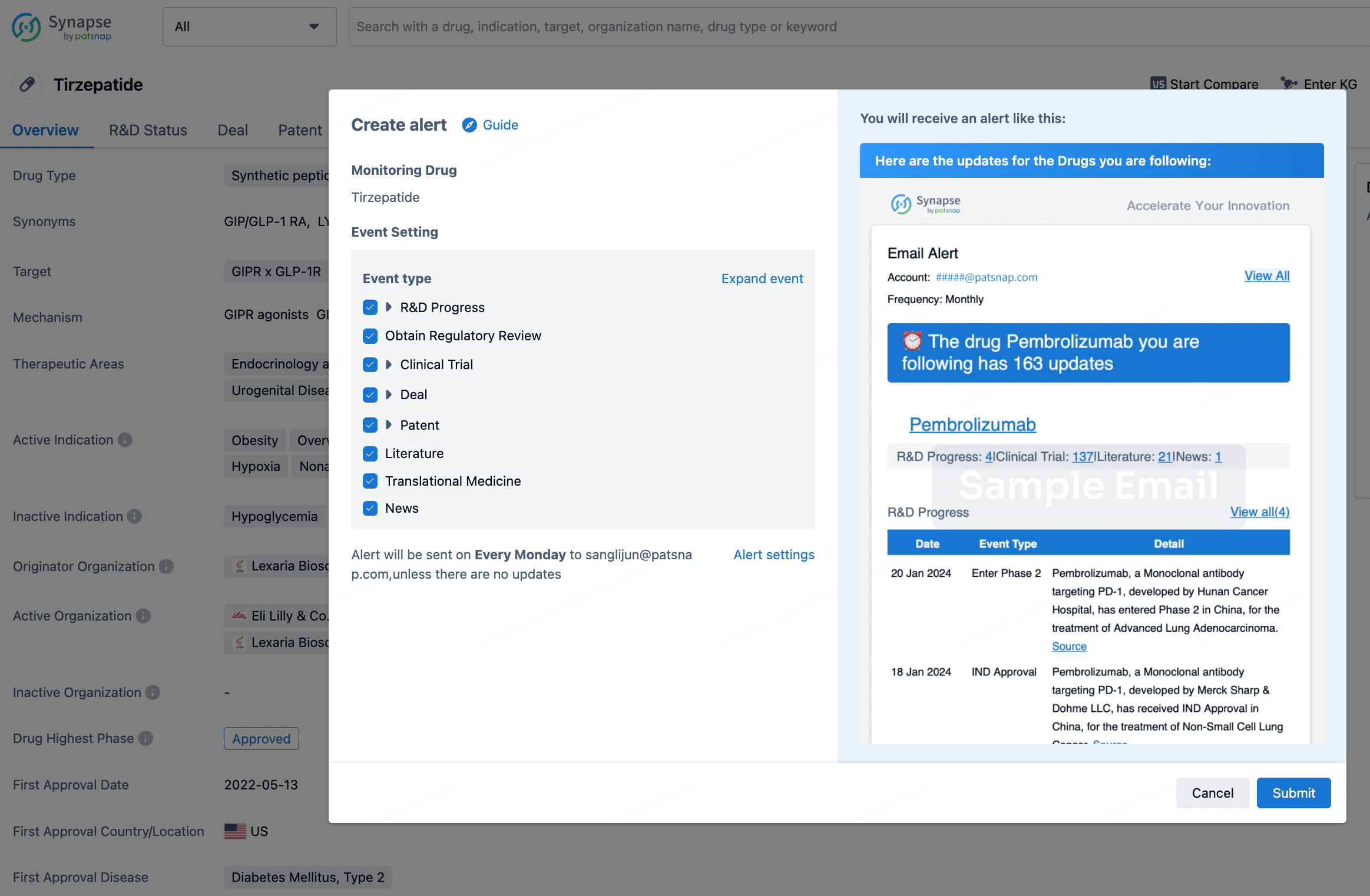
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!