The Swiss company Confo has completed a series B financing of 60 million euros to advance multiple projects, including GPR75
On July 26, Belgian biotech company Confo Therapeutics (Confo) announced that it has completed a €60 million Series B financing round. This round was led by Ackermans & van Haaren (AvH), and received participation from new investors Driehaus Capital Management and Quest for Growth (QfG), alongside existing investors BioGeneration Ventures (BGV), Capricorn Health-tech Fund (CHF), Fund+, MINTS, Perceptive Advisors, Qbic, PMV, V-Bio Ventures, VIB, and Wellington Partners. The funding will be used to advance two wholly owned projects into Phase I clinical trials, and move two other projects to the Investigational New Drug (IND) approval stage, including the GPR75 target molecule for obesity.
Confo is a platform-based biotech company focused on the discovery and development of GPCR class drug targets. At the beginning of 2023, Confo announced a global licensing agreement with Eli Lilly on the clinical treatment of neuropathic pain related to their investigational drug CFTX-1554 and alternative compounds, jointly advancing the AT2R antagonist drug.
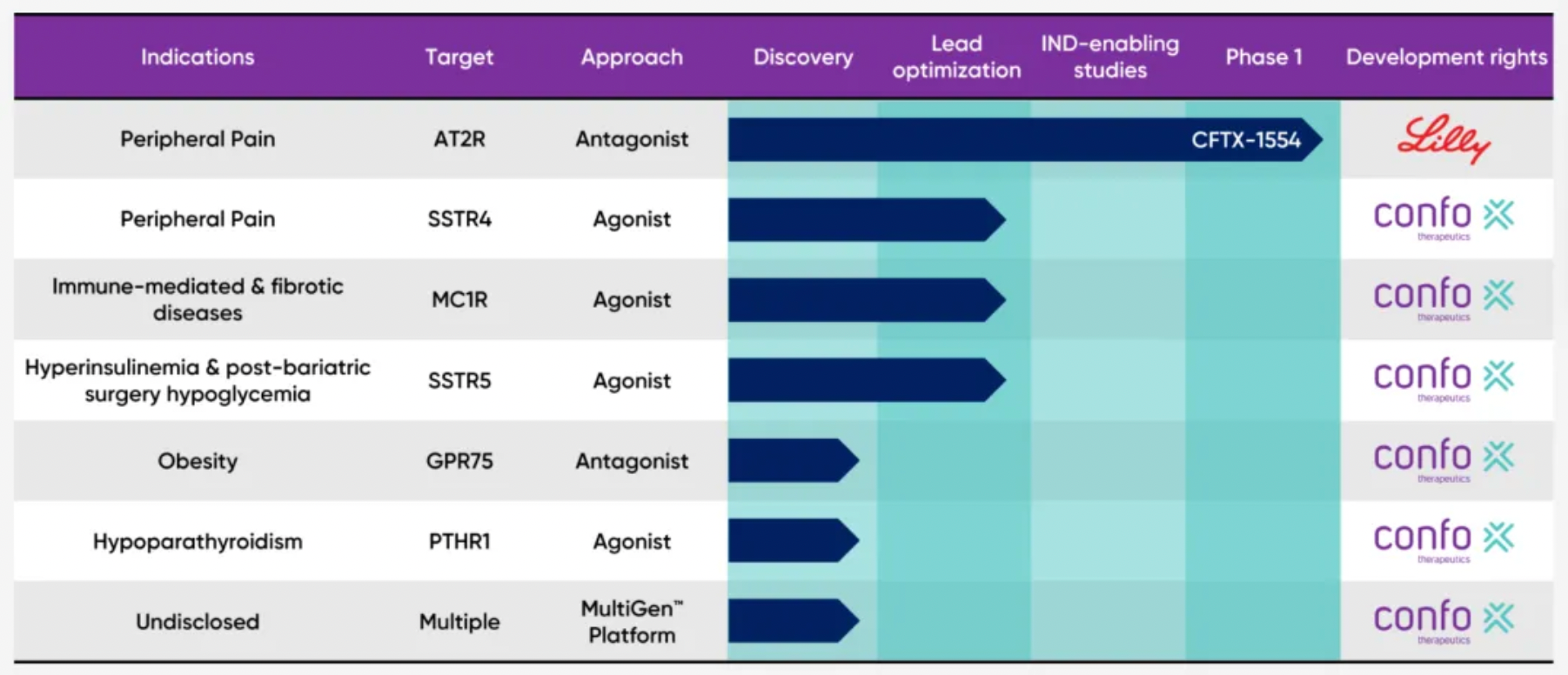
The current status of the company's pipeline is as follows:
Confo Therapeutics is a Belgian company specializing in new drug discovery on GPCR family targets. The company possesses a proprietary drug discovery engine (accurately locking in the required GPCR conformation using nanobodies), this unique ability allows Confo to tap into vast untapped potentialities, discovering and developing breakthrough drugs.
The company's main technology route is as follows:
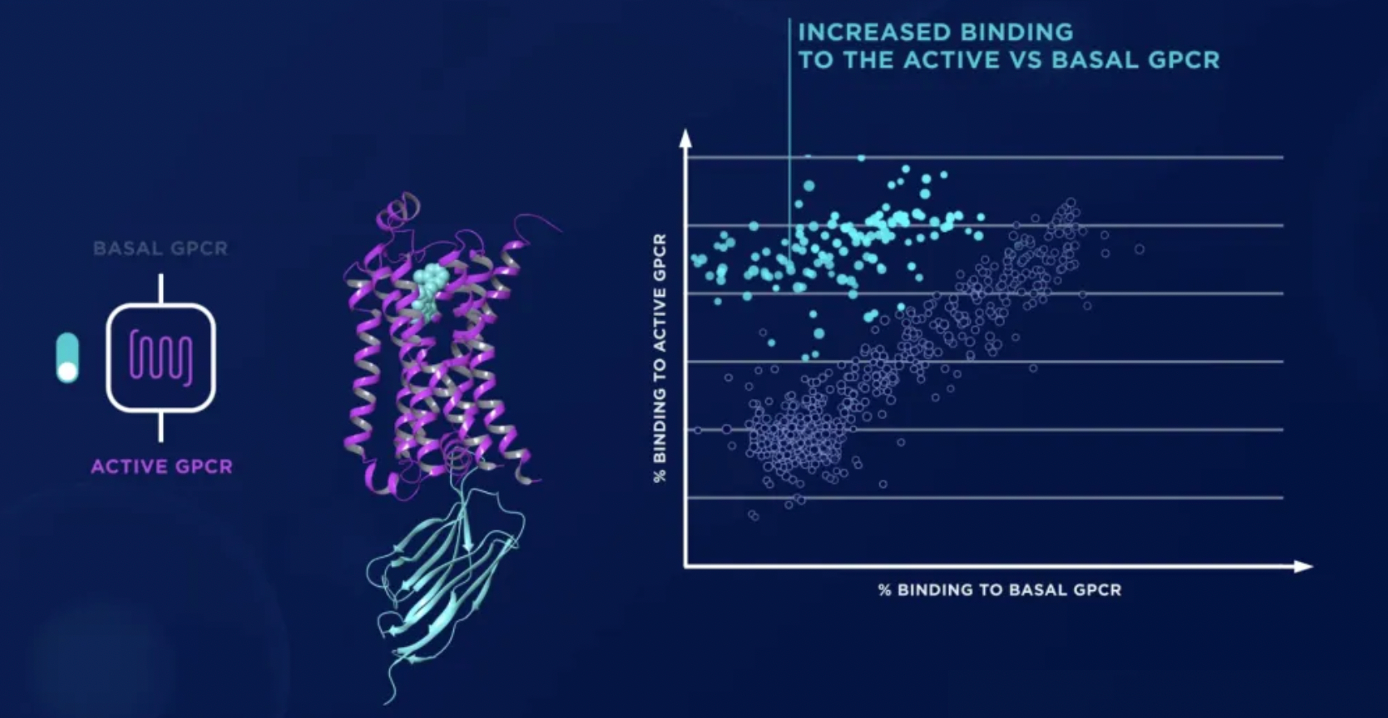
ConfoBody can stably keep GPCR in its active conformation, thereby increasing the chances of identifying ligands with conformational selectivity. For example, the active state-specific ConfoBody can stabilize the agonist-GPCR complex, enhancing sensitivity to the agonist. The proprietary ConfoScreen method has a high sensitivity, capable of identifying smaller chemical starting points, including compound fragments with a molecular weight of <300 MW.
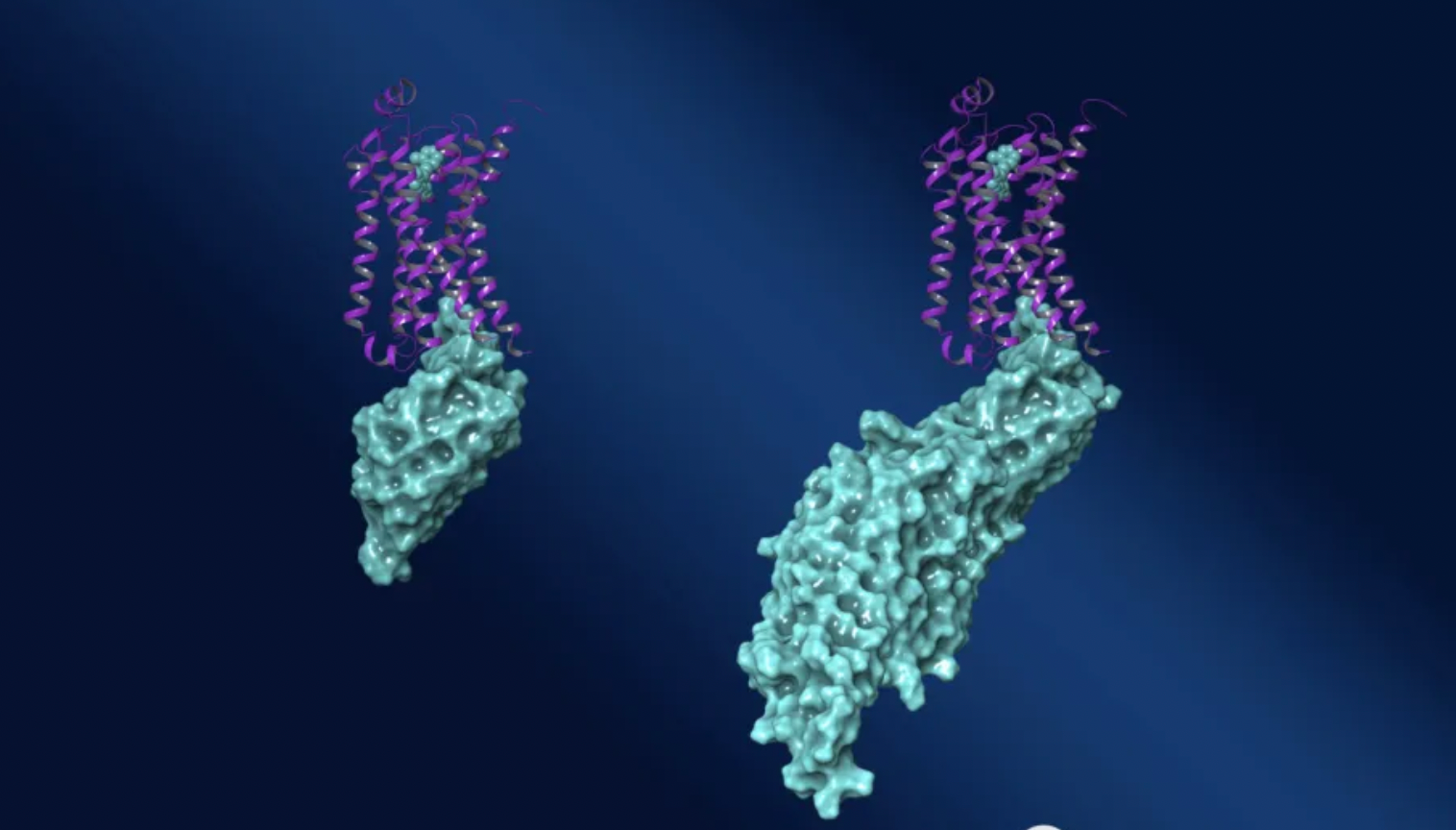
By developing ConfoBody that specifically recognizes receptors, on one hand, it can stabilize the conformation of the receptor in the expected state, on the other hand, some nanobodies can also stabilize the receptor-G protein (mainly Gs subtype) complex.
The company has obtained the exclusive license for MegaBody™ technology, which is a new scaffold based on ConfoBody. It retains the conformational selectivity of the parent ConfoBody, while providing more mass and structural features for high-resolution cryo-electron microscopy. MegaBodies™ are able to capture protein conformations, influence particle orientation, serve as a handle for three-dimensional image reconstruction, and increase the size of protein complexes, thus making GPCR more suitable for study through cryo-electron microscopy.
With ConfoStructures, a method based on structure-based drug design is used to quickly and efficiently improve µM chemical starting points into nM affinity lead compounds, and endow them with excellent drug-like characteristics.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!